Abstract
Background
Spinal and bulbar muscular atrophy (SBMA) is an adult-onset, hereditary neuromuscular disease characterized by muscle atrophy, weakness, contraction fasciculation, and bulbar involvement. Although the causative gene, androgen receptor, has been identified, the development of novel therapeutics for SBMA is incomplete. In this study, the efficacy and safety of leuprorelin acetate administration for patients with SBMA, using the pooled data of two randomized-controlled trials, was studied.
Methods
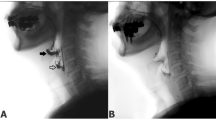
Two randomized double-blinded studies (JASMITT-06DB and JASMITT-11DB) were done as multicentric, investigator-initiated clinical trials in Japan. In both studies, eligible patients were randomly assigned 1:1 to receive leuprorelin acetate administration once per 12 weeks for 48 weeks. The primary endpoint was the longitudinal change of pharyngeal barium residues from the baseline data measured with videofluorographic swallowing analyses. The pooled analysis plan was decided upon after the 06B study was finished and before the 11DB study began.
Results
The primary endpoint difference between the leuprorelin group and the placebo group was pharyngeal barium residue after initial swallowing, − 4.12% (95% CI, − 8.40–0.15; p = 0.058). The primary endpoint of this study does not reach significant results, although inter-group differences of pharyngeal barium residues after the initial swallowing indicated that leuprorelin acetate may be effective at each assessment point in both study groups.
Conclusions
The efficacy of leuprorelin acetate for patients with SBMA was statistically similar in two randomized-controlled trials, and suggested that leuprorelin acetate may be effective and safe. Further investigations are needed to clarify the promising efficacy of the drug.


Similar content being viewed by others
References
Kennedy WR, Alter M, Sung JH (1968) Progressive proximal spinal and bulbar muscular atrophy of late onset. A wsex-linked recessive trait. Neurology 18:671–680
Sobue G, Hashizume Y, Mukai E et al (1989) X-linked recessive bulbospinal neuronopathy. A clinicopathological study. Brain 112:209–232
Sperfeld AD, Karitzky J, Brummer D et al (2002) X-linked bulbospinal neuronopathy: Kennedy disease. Arch Neurol 59:1921–1926
Fischbeck KH (1997) Kennedy disease. J Inherit Metab Dis 20:152–158
Atsuta N, Watanabe H, Ito M et al (2006) Natural history of spinal and bulbar muscular atrophy (SBMA): a study of 223 Japanese patients. Brain 129:1446–1455
Chevalier-Larsen ES, O’Brien CJ, Wang H et al (2004) Castration restores function and neurofilament alterations of aged symptomatic males in a transgenic mouse model of spinal and bulbar muscular atrophy. J Neurosci 19(24):4778–4786
Katsuno M, Adachi H, Kume A et al (2002) Testosterone reduction prevents phenotypic expression in a transgenic mouse model of spinal and bulbar muscular atrophy. Neuron 35:843–854
Katsuno M, Adachi H, Doyu M et al (2003) Leuprorelin rescues polyglutamine-dependent phenotypes in a transgenic mouse model of spinal and bulbar muscular atrophy. Nat Med 9:768–773
Banno H, Katsuno M, Suzuki K et al (2009) Phase 2 trial of leuprorelin in patients with spinal and bulbar muscular atrophy. Ann Neurol 65:140–150
Katsuno M, Banno H, Suzuki K et al (2010) Efficacy and safety of leuprorelin in patients with spinal and bulbar muscular atrophy (JASMITT study): a multicentre, randomised, double-blind, placebo-controlled trial. Lancet Neurol 9:875–884
Fernández-Rhodes LE1, Kokkinis AD, White MJ et al (2011) Efficacy and safety of dutasteride in patients with spinal and bulbar muscular atrophy: a randomised placebo-controlled trial. Lancet Neurol 10:140–147
Mitsumoto H, Brooks BR, Silani V (2014) Clinical trials in amyotrophic lateral sclerosis: why so many negative trials and how can trials be improved. Lancet Neurol 13:1127–1138
Logemann JA, Pauloski BR, Rademaker AW, Colangelo LA, Kahrilas PJ, Smith CH (2000) Temporal and biomechanical characteristics of oropharyngeal swallow in younger and older men. J Speech Lang Hear Res 43:1264–1274
The ALS CNTF treatment study (ACTS) phase I-II Study Group (1996) The Amyotrophic Lateral Sclerosis Functional Rating Scale. Assessment of activities of daily living in patients with amyotrophic lateral sclerosis. Arch Neurol 53:141–147
Ohashi Y, Tashiro K, Itoyama Y, Nakano I, Sobue G, Nakamura S et al (2001) Study of functional rating scale for amyotrophic lateral sclerosis: revised ALSFRS (ALSFRS-R) Japanese version. No To Shinkei 53:346–355
Besinger UA, Toyka KV, Hömberg M, Heininger K, Hohlfeld R, Fateh-Moghadam A (1983) Myasthenia gravis: long-term correlation of binding and bungarotoxin blocking antibodies against acetylcholine receptors with changes in disease severity. Neurology 33:1316–1321
Jenkinson C, Fitzpatrick R, Brennan C, Bromberg M, Swash M (1999) Development and validation of a short measure of health status for individuals with amyotrophic lateral sclerosis/motor neurone disease: the ALSAQ-40. J Neurol 246(Suppl 3):III16–I21
Jenkinson C, Fitzpatrick R (2001) Reduced item set for the amyotrophic lateral sclerosis assessment questionnaire: development and validation of the ALSAQ-5. J Neurol Neurosurg Psychiatry 70:70–73
Yamaguchi T, Ohbu S, Saito M, Ito Y, Morikawa F, Tashiro K et al (2004) Validity and clinical applicability of the Japanese version of amyotrophic lateral sclerosis assessment questionnaire 40 (ALSAQ-40). No To Shinkei 56:483–494
ICH guideline E2B (R2) (2001) Electronic transmission of individual case safety reports—message specification (ICH ICSR DTD Version 2.1). Final Version 2.3, Document Revision 1 Feb 2001
Kelly PJ, Albers GW, Chatzikonstantinou A et al (2016) Validation and comparison of imaging-based scores for prediction of early stroke risk after transient ischaemic attack: a pooled analysis of individual-patient data from cohort studies. Lancet Neurol 15:1238–1247
Anderson C, Teo K, Gao P et al (2011) Renin-angiotensin system blockade and cognitive function in patients at high risk of cardiovascular disease: analysis of data from the ONTARGET and TRANSCEND studies. Lancet Neurol 10:43–53
Vahedi K, Hofmeijer J, Juettler E et al (2007) Early decompressive surgery in malignant infarction of the middle cerebral artery: a pooled analysis of three randomised controlled trials. Lancet Neurol 6:215–222
Johansson E, Cuadrado-Godia E, Hayden D et al (2016) Recurrent stroke in symptomatic carotid stenosis awaiting revascularization: a pooled analysis. Neurology 86:498–504
Sperling MR, French J, Jacobson MP et al (2016) Conversion to eslicarbazepine acetate monotherapy: A pooled analysis of 2 phase III studies. Neurology 86:1095–1102
Banno H, Katsuno M, Suzuki K et al (2017) Swallowing markers in spinal and bulbar muscular atrophy. Ann Clin Transl Neurol 24(4):534–543
Hashizume A, Banno H, Katsuno M et al (2017) Quantitative assessment of swallowing dysfunction in patients with spinal and bulbar muscular atrophy. Intern Med 56:3159–3165
Li M, Miwa S, Kobayashi Y et al (1998) Nuclear inclusions of the androgen receptor protein in spinal and bulbar muscular atrophy. Ann Neurol 44:249–254
Banno H, Adachi H, Katsuno M et al (2006) Mutant androgen receptor accumulation in spinal and bulbar muscular atrophy scrotal skin: a pathogenic marker. Ann Neurol 59:520–526
Wasserstein RL, Lazar NA (2016) The ASA’s statement on p-values: context, process, and purpose. Am Stat 70:129–133
Acknowledgements
This study is funded by Large Scale Clinical Trial Network Project, which is subsidised by the Japan Ministry of Health, Labour and Welfare and supported by Health and Labour Sciences Research Grants, Japan. Study drugs were provided by Takeda Pharmaceuticals. We thank all patients and their families for participating in this study. JASMITT study group: Principle investigator in 06 DB trial, H Sasaki, M Aoki, I Nakano, S Ito, H Mizusawa, To Yamamoto, K Hasegawa, H Miyajima, G Sobue, N Kanda, K Nakajima, A tsujino, M Uchino, Principle investigator in 11DB trial, M Morita, K Kanai, Ta Yamamoto, H Mizusawa, To Yamamoto, and G Sobue.
Author information
Authors and Affiliations
Consortia
Contributions
AH drafting/revising the manuscript, analysis/interpretation of the data, research project execution, data acquisition, statistical analysis, and study design and concept. MK research project organization, research project execution, revising the manuscript, interpretation of the data, statistical analysis, study design and concept. KS acquisition of data, research project execution, and study design and concept. HB acquisition of data, research project execution, and study design and concept. YT, MK, NS, TM, AA, and YH: acquisition of data and research project execution. GS: research project organization, research project execution, revising the manuscript, interpretation of the data, statistical analysis, and study design and concept.
Corresponding authors
Ethics declarations
Conflicts of interest
MK and GS have received honoraria from Takeda Pharmaceuticals. All other authors have no conflicts of interest.
Additional information
Members of the group “JASMITT study group” are listed in Acknowledgements section.
Rights and permissions
About this article
Cite this article
Hashizume, A., Katsuno, M., Suzuki, K. et al. Efficacy and safety of leuprorelin acetate for subjects with spinal and bulbar muscular atrophy: pooled analyses of two randomized-controlled trials. J Neurol 266, 1211–1221 (2019). https://doi.org/10.1007/s00415-019-09251-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-019-09251-x