Abstract
Objective
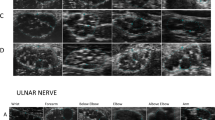
HRUS is increasingly being used in the diagnosis and evaluation of autoimmune neuropathies such as CIDP. Recently, studies focused not only on changes of nerves size, but also the fascicular structure and the echogenicity changes in CIDP. However, little is known about the alterations of echogenicity in the long-term course in CIDP. The aim of this study was to evaluate echogenicity in CIDP patients in a long-term follow-up period and to analyze the benefit of the evaluation of echogenicity compared to nerve size.
Methods
20 patients fulfilling the definite diagnostic criteria of CIDP received clinical examination, nerve conduction studies and HRUS every 6 months over a median follow-up time of 34 months. Patients were divided into clinically stable/regressive disease course or progressive disease course according to the development of the inflammatory neuropathy cause and treatment overall disability sum score. Echogenicity of peripheral nerves was measured semi-automated and quantitative. Echogenicity was divided into three classes by fraction of black: hypoechogenic, mixed hypo-/hyperechogenic, hyperechogenic.
Results
Patients with hyperechogenic arm nerves more frequently show clinical worsening, whereas patients with hypoechogenic arm nerves remain stable or even improved over time. In the long-term course of the disease, echogenicity mostly did not change, and if changes occured echogenicity did not correspond to ODSS changes.
Conclusion
Echogenicity of the arm nerves in CIDP may be used as a prognostic marker, but not as a follow-up tool for evaluating clinical changes. Further studies in a larger cohort are needed to confirm these results.






Similar content being viewed by others
References
Grimm A, Décard BF, Axer H, Fuhr P (2015) The Ultrasound pattern sum score—UPSS. A new method to differentiate acute and subacute neuropathies using ultrasound of the peripheral nerves. Clin Neurophysiol 126:2216–2225. https://doi.org/10.1016/j.clinph.2015.01.011
Kerasnoudis A, Pitarokoili K, Behrendt V et al (2014) Nerve ultrasound score in distinguishing chronic from acute inflammatory demyelinating polyneuropathy. Clin Neurophysiol 125:635–641. https://doi.org/10.1016/j.clinph.2013.08.014
Padua L, Martinoli C, Pazzaglia C et al (2012) Intra- and internerve cross-sectional area variability: new ultrasound measures. Muscle Nerve 45:730–733. https://doi.org/10.1002/mus.23252
Padua L, Granata G, Sabatelli M et al (2013) Heterogeneity of root and nerve ultrasound pattern in CIDP patients. Clin Neurophysiol 125:1–6. https://doi.org/10.1016/j.clinph.2018.03.036
Härtig F, Ross M, Dammeier NM et al (2018) Nerve ultrasound predicts treatment response in chronic inflammatory demyelinating polyradiculoneuropathy—a prospective follow-up. Neurotherapeutics 15:439–451. https://doi.org/10.1007/s13311-018-0609-4
Fisse AL, Pitarokoili K, Trampe N et al (2018) Clinical, sonographic, and electrophysiologic longitudinal features of chronic inflammatory demyelinating polyneuropathy. J Neuroimaging 9:402. https://doi.org/10.1111/jon.12579
Van den Bergh PYK, Hadden RDM, Bouche P et al (2010) European Federation of Neurological Societies/Peripheral Nerve Society Guideline on management of chronic inflammatory demyelinating polyradiculoneuropathy: report of a joint task force of the European Federation of Neurological Societies and the Peripheral Nerve Society—First Revision. Eur J Neurol 17:356–363. https://doi.org/10.1111/j.1468-1331.2009.02930.x
Merkies ISJ, Schmitz PIM, van der Meche FGA et al (2002) Clinimetric evaluation of a new overall disability scale in immune mediated polyneuropathies. J Neurol Neurosurg Psychiatr 72:596–601. https://doi.org/10.1136/jnnp.72.5.596
Boom J, Visser LH (2012) Quantitative assessment of nerve echogenicity: comparison of methods for evaluating nerve echogenicity in ulnar neuropathy at the elbow. Clin Neurophysiol 123:1446–1453. https://doi.org/10.1016/j.clinph.2011.10.050
Dwyer MG, Bergsland N, Ramasamy DP et al (2018) Atrophied brain lesion volume: a new imaging biomarker in multiple sclerosis. J Neuroimaging 11:597. https://doi.org/10.1111/jon.12527
Magliozzi R, Reynolds R, Calabrese M (2018) MRI of cortical lesions and its use in studying their role in MS pathogenesis and disease course. Brain Pathol. https://doi.org/10.1111/bpa.12642
Goedee HS, van der Pol WL, van Asseldonk J-TH et al (2017) Diagnostic value of sonography in treatment-naive chronic inflammatory neuropathies. Neurology 88:143–151. https://doi.org/10.1212/WNL.0000000000003483
Décard BF, Pham M, Grimm A (2018) Ultrasound and MRI of nerves for monitoring disease activity and treatment effects in chronic dysimmune neuropathies—current concepts and future directions. Clin Neurophysiol 129:155–167. https://doi.org/10.1016/j.clinph.2017.10.028
Kerasnoudis A, Pitarokoili K, Gold R, Yoon M-S (2015) Nerve ultrasound and electrophysiology for therapy monitoring in chronic inflammatory demyelinating polyneuropathy. J Neuroimaging 25:931–939. https://doi.org/10.1111/jon.12279
Author information
Authors and Affiliations
Contributions
ALF: Study design, data collection, drafting and revising the manuscript. KP: Study design, data collection, drafting and revising the manuscript. DG: Data collection, drafting and revising the manuscript. JM: Data collection, drafting and revising the manuscript. AK: Data collection, drafting and revising the manuscript. RG: Critical comments during data collection and manuscript revision. M-SY: Study design, critical comments during data collection and manuscript revision.
Corresponding author
Ethics declarations
Conflicts of interest
Anna Lena Fisse: none. Kalliopi Pitarokoili: received travel grants and speakers’ honoraria from Novartis, Biogen idec, Teva, Bayer and Grifols all not related to the manuscript. Donata Gamber: none. Jeremias Motte: received travel grants from Biogen idec. Antonios Kerasnoudis: received travel grants and speakers’ honoraria from Grifols and Genesis all not related to the manuscript. Ralf Gold: received consultation fees and speaker honoraria from Bayer Schering, Biogen idec, Merck Serono, Novartis, Sanofi-Aventis and TEVA. He also acknowledges grant support from Bayer Schering, Biogen idec, Merck Serono, Sanofi-Aventis and TEVA, none related to this manuscript. Min-Suk Yoon: Dr. Yoon received speakers’ honoraria from CSL Behring, Grifols and scientific grant from CSL Behring.
Ethical standards
The ethics committee of the medical faculty of Ruhr University Bochum approved the study protocol and all patients signed informed consent (vote no. 4382-12, ethics committee of the medical faculty of the Ruhr University Bochum).
Rights and permissions
About this article
Cite this article
Fisse, A.L., Pitarokoili, K., Motte, J. et al. Nerve echogenicity and intranerve CSA variability in high-resolution nerve ultrasound (HRUS) in chronic inflammatory demyelinating polyneuropathy (CIDP). J Neurol 266, 468–475 (2019). https://doi.org/10.1007/s00415-018-9158-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-018-9158-3