Abstract
Background
Natalizumab (NTZ) was the first approved monoclonal antibody for the treatment of relapsing-remitting multiple sclerosis (RRMS). Despite proven and sustained efficacy, its use is limited by the risk of progressive multifocal leukoencephalopathy (PML). Moreover, some patients show ongoing disease activity under NTZ, requiring a switch to another disease-modifying treatment (DMT). However, evidence regarding the optimal DMT for treatment of active RRMS after NTZ-cessation is still scarce.
Objective
To evaluate efficacy and safety outcomes of ALEM vs FTY treatment after cessation of NTZ.
Methods
We retrospectively identified patients at 12 German neurology centers and analyzed risks for disease activity, adverse events, disability progression, and treatment discontinuation.
Results
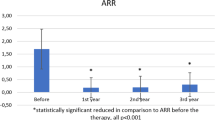
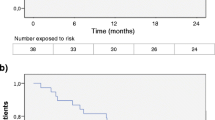
195 patients were identified and 144 underwent final analysis (FTY: 101; ALEM: 42). The hazard ratio for clinical relapses was 2.24 favoring ALEM (95% CI 1.12–4.50; p = 0.015). The hazard ratio for adverse events was 7.78 (95% CI 1.04–57.95; p = 0.006) and 2.41 for MRI progression (95% CI 1.26–4.60; p = 0.004). The odds ratio for disability progression after 12 months was 4.84 (95% CI 1.74–13.47, p = 0.003). Differences remained after adjusting for possible confounders (e.g., age, sex, baseline disability, NTZ treatment duration, washout time).
Conclusion
Our findings indicated particular advantages of ALEM compared to FTY in patients stopping NTZ.


Similar content being viewed by others
References
Polman CH, O’Connor PW, Havrdova E, Hutchinson M, Kappos L, Miller DH, Phillips JT, Lublin FD, Giovannoni G, Wajgt A, Toal M, Lynn F, Panzara MA, Sandrock AW, Investigators A (2006) A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med 354(9):899–910. https://doi.org/10.1056/NEJMoa044397
Rudick RA, Stuart WH, Calabresi PA, Confavreux C, Galetta SL, Radue EW, Lublin FD, Weinstock-Guttman B, Wynn DR, Lynn F, Panzara MA, Sandrock AW, Investigators S (2006) Natalizumab plus interferon beta-1a for relapsing multiple sclerosis. N Engl J Med 354(9):911–923. https://doi.org/10.1056/NEJMoa044396
Bloomgren G, Richman S, Hotermans C, Subramanyam M, Goelz S, Natarajan A, Lee S, Plavina T, Scanlon JV, Sandrock A, Bozic C (2012) Risk of natalizumab-associated progressive multifocal leukoencephalopathy. N Engl J Med 366(20):1870–1880. https://doi.org/10.1056/NEJMoa1107829
Schwab N, Schneider-Hohendorf T, Melzer N, Cutter G, Wiendl H (2017) Natalizumab-associated PML: challenges with incidence, resulting risk, and risk stratification. Neurology. https://doi.org/10.1212/WNL.0000000000003739
Kappos L, Bates D, Edan G, Eraksoy M, Garcia-Merino A, Grigoriadis N, Hartung HP, Havrdova E, Hillert J, Hohlfeld R, Kremenchutzky M, Lyon-Caen O, Miller A, Pozzilli C, Ravnborg M, Saida T, Sindic C, Vass K, Clifford DB, Hauser S, Major EO, O’Connor PW, Weiner HL, Clanet M, Gold R, Hirsch HH, Radu EW, Sorensen PS, King J (2011) Natalizumab treatment for multiple sclerosis: updated recommendations for patient selection and monitoring. Lancet Neurol 10(8):745–758. https://doi.org/10.1016/S1474-4422(11)70149-1
Derfuss T, Kovarik JM, Kappos L, Savelieva M, Chhabra R, Thakur A, Zhang Y, Wiendl H, Tomic D (2017) alpha4-integrin receptor desaturation and disease activity return after natalizumab cessation. Neurol Neuroimmunol Neuroinflamm 4(5):e388. https://doi.org/10.1212/NXI.0000000000000388
Miravalle A, Jensen R, Kinkel RP (2011) Immune reconstitution inflammatory syndrome in patients with multiple sclerosis following cessation of natalizumab therapy. Arch Neurol 68(2):186–191. https://doi.org/10.1001/archneurol.2010.257
Iaffaldano P, Lucisano G, Pozzilli C, Brescia Morra V, Ghezzi A, Millefiorini E, Patti F, Lugaresi A, Zimatore GB, Marrosu MG, Amato MP, Bertolotto A, Bergamaschi R, Granella F, Coniglio G, Tedeschi G, Sola P, Lus G, Ferro MT, Iuliano G, Corea F, Protti A, Cavalla P, Guareschi A, Rodegher M, Paolicelli D, Tortorella C, Lepore V, Prosperini L, Sacca F, Baroncini D, Comi G, Trojano M, Italian iMed-Web d (2015) Fingolimod versus interferon beta/glatiramer acetate after natalizumab suspension in multiple sclerosis. Brain 138 (Pt 11):3275–3286. https://doi.org/10.1093/brain/awv260
Kappos L, Radue EW, Comi G, Montalban X, Butzkueven H, Wiendl H, Giovannoni G, Hartung HP, Derfuss T, Naegelin Y, Sprenger T, Mueller-Lenke N, Griffiths S, von Rosenstiel P, Gottschalk R, Zhang Y, Dahlke F, Tomic D, group Ts (2015) Switching from natalizumab to fingolimod: a randomized, placebo-controlled study in RRMS. Neurology 85(1):29–39. https://doi.org/10.1212/WNL.0000000000001706
Alping P, Frisell T, Novakova L, Islam-Jakobsson P, Salzer J, Bjorck A, Axelsson M, Malmestrom C, Fink K, Lycke J, Svenningsson A, Piehl F (2016) Rituximab versus fingolimod after natalizumab in multiple sclerosis patients. Ann Neurol 79(6):950–958. https://doi.org/10.1002/ana.24651
Investigators CT, Coles AJ, Compston DA, Selmaj KW, Lake SL, Moran S, Margolin DH, Norris K, Tandon PK (2008) Alemtuzumab vs. interferon beta-1a in early multiple sclerosis. N Engl J Med 359(17):1786–1801. https://doi.org/10.1056/NEJMoa0802670
Coles AJ, Twyman CL, Arnold DL, Cohen JA, Confavreux C, Fox EJ, Hartung HP, Havrdova E, Selmaj KW, Weiner HL, Miller T, Fisher E, Sandbrink R, Lake SL, Margolin DH, Oyuela P, Panzara MA, Compston DA, investigators C-MI (2012) Alemtuzumab for patients with relapsing multiple sclerosis after disease-modifying therapy: a randomised controlled phase 3 trial. Lancet 380(9856):1829–1839. https://doi.org/10.1016/S0140-6736(12)61768-1
Costelloe L, Jones J, Coles A (2012) Secondary autoimmune diseases following alemtuzumab therapy for multiple sclerosis. Expert Rev Neurother 12(3):335–341. https://doi.org/10.1586/ern.12.5
Ruck T, Bittner S, Wiendl H, Meuth SG (2015) Alemtuzumab in multiple sclerosis: mechanism of action and beyond. Int J Mol Sci 16(7):16414–16439. https://doi.org/10.3390/ijms160716414
Malucchi S, Capobianco M, Lo Re M, Malentacchi M, di Sapio A, Matta M, Sperli F, Bertolotto A (2016) High-risk PML patients switching from natalizumab to alemtuzumab: an observational study. Neurol Ther. https://doi.org/10.1007/s40120-016-0058-0
Kalincik T, Brown JWL, Robertson N, Willis M, Scolding N, Rice CM, Wilkins A, Pearson O, Ziemssen T, Hutchinson M, McGuigan C, Jokubaitis V, Spelman T, Horakova D, Havrdova E, Trojano M, Izquierdo G, Lugaresi A, Prat A, Girard M, Duquette P, Grammond P, Alroughani R, Pucci E, Sola P, Hupperts R, Lechner-Scott J, Terzi M, Van Pesch V, Rozsa C, Grand’Maison F, Boz C, Granella F, Slee M, Spitaleri D, Olascoaga J, Bergamaschi R, Verheul F, Vucic S, McCombe P, Hodgkinson S, Sanchez-Menoyo JL, Ampapa R, Simo M, Csepany T, Ramo C, Cristiano E, Barnett M, Butzkueven H, Coles A, Group MSS (2017) Treatment effectiveness of alemtuzumab compared with natalizumab, fingolimod, and interferon beta in relapsing-remitting multiple sclerosis: a cohort study. Lancet Neurol 16(4):271–281. https://doi.org/10.1016/S1474-4422(17)30007-8
Willis M, Pearson O, Illes Z, Sejbaek T, Nielsen C, Duddy M, Petheram K, van Munster C, Killestein J, Malmestrom C, Tallantyre E, Robertson N (2017) An observational study of alemtuzumab following fingolimod for multiple sclerosis. Neurol Neuroimmunol Neuroinflamm 4(2):e320. https://doi.org/10.1212/NXI.0000000000000320
Wing MG, Moreau T, Greenwood J, Smith RM, Hale G, Isaacs J, Waldmann H, Lachmann PJ, Compston A (1996) Mechanism of first-dose cytokine-release syndrome by CAMPATH 1-H: involvement of CD16 (FcgammaRIII) and CD11a/CD18 (LFA-1) on NK cells. J Clin Invest 98(12):2819–2826. https://doi.org/10.1172/JCI119110
Cohen JA, Coles AJ, Arnold DL, Confavreux C, Fox EJ, Hartung HP, Havrdova E, Selmaj KW, Weiner HL, Fisher E, Brinar VV, Giovannoni G, Stojanovic M, Ertik BI, Lake SL, Margolin DH, Panzara MA, Compston DA, investigators C-MI (2012) Alemtuzumab versus interferon beta 1a as first-line treatment for patients with relapsing-remitting multiple sclerosis: a randomised controlled phase 3 trial. Lancet 380(9856):1819–1828. https://doi.org/10.1016/S0140-6736(12)61769-3
Giovannoni G, Marta M, Davis A, Turner B, Gnanapavan S, Schmierer K (2016) Switching patients at high risk of PML from natalizumab to another disease-modifying therapy. Pract Neurol 16(5):389–393. https://doi.org/10.1136/practneurol-2015-001355
Leurs CE, van Kempen ZL, Dekker I, Balk LJ, Wattjes MP, Rispens T, Uitdehaag BM, Killestein J (2017) Switching natalizumab to fingolimod within 6 weeks reduces recurrence of disease activity in MS patients. Mult Scler. https://doi.org/10.1177/1352458517726381
Acknowledgements
The study was financially supported by Sanofi Genzyme (ALAIN01 to Sven G. Meuth and Tobias Ruck) and the Competence Network Multiple Sclerosis (01GI1603D, PROGRAMMS to Sven G. Meuth and Heinz Wiendl).
Funding
The study was financially supported by Sanofi Genzyme (ALAIN01 to Sven G. Meuth and Tobias Ruck) and the Competence Network Multiple Sclerosis (01GI1603D, PROGRAMMS to Sven G. Meuth and Heinz Wiendl).
Author information
Authors and Affiliations
Contributions
SP: study concept and design, acquisition of data, analysis and interpretation of data, writing of the manuscript. RS: analysis and interpretation of data, writing of the manuscript. FAS: acquisition of data, analysis and interpretation of data. RP: acquisition of data, critical revision of manuscript for intellectual content. CK: acquisition of data, critical revision of manuscript for intellectual content. MW: acquisition of data. DL: acquisition of data, critical revision of manuscript for intellectual content. RL: acquisition of data, critical revision of manuscript for intellectual content. SD: acquisition of data, critical revision of manuscript for intellectual content. VS: acquisition of data, critical revision of manuscript for intellectual content. SW: acquisition of data, critical revision of manuscript for intellectual content. MP: acquisition of data, critical revision of manuscript for intellectual content. CA: acquisition of data, critical revision of manuscript for intellectual content. ML: acquisition of data, critical revision of manuscript for intellectual content. CE: acquisition of data, critical revision of manuscript for intellectual content. BT: acquisition of data, critical revision of manuscript for intellectual content. VL: acquisition of data, critical revision of manuscript for intellectual content. BW: acquisition of data, critical revision of manuscript for intellectual content. JH: acquisition of data, critical revision of manuscript for intellectual content. LK: acquisition of data, critical revision of manuscript for intellectual content. HW: critical revision of manuscript for intellectual content. TR: study concept and design, acquisition of data, analysis and interpretation of data, critical revision of manuscript for intellectual content. SGM: study concept and design, analysis and interpretation of data, critical revision of manuscript for intellectual content.
Corresponding author
Ethics declarations
Conflicts of interest
Steffen Pfeuffer: received travel reimbursements from Sanofi-Genzyme and Merck and honoraria for lecturing from Sanofi Genzyme, Biogen and Mylan. Rene Schmidt: declares no conflicts of interest. Frederike Anne Straeten: declares no conflicts of interest. Refik Pul: received travel reimbursements from Merck, research support by Novartis, speaker honoraria from Merck Serono, Biogen, Novartis, Sanofi-Genzyme, Mylan and Roche. Christoph Kleinschnitz: received travel reimbursements, honoraria for lecturing and research support from Ablynx, Amgen, Bayer Vital, Bristol-Mayers Squibb, Biotronik, Boehringer Ingelheim, Biogen, CSL Behring, Daiichi-Sankyo, Desitin, Eisai, Ever Pharma, Sanofi Genzyme, Merck Serono, Mylan, Medday, Novartis, Pfizer, Roche, Siemens, Stago and Teva. Marinus Wieshuber: declares no conflict of interest. De-Hyung Lee: received compensation for activities with Biogen, Merck, Novartis, Roche, and Sanofi-Genzyme. Ralf A. Linker: received compensation for activities with Biogen, Merck, Novartis, Roche, and Sanofi-Genzyme. Sebastian Doerck: reports no conflicts of interest. Vera Straeten: received travel reimbursements from Novartis, Merck and Biogen and honoraria for lecturing from Sanofi Genzyme, Biogen and Novartis. Susanne Windhagen: declares no conflict of interest. Marc Pawlitzki: received honoraria for lecturing and travel reimbursements from Biogen, Sanofi Genzyme, Merck Serono, Roche and Novartis. Christoph Aufenberg: received travel reimbursements from Bayer. Michael Lang: received travel grants, honoraria for lecturing, financial research support and consultancy fees from Teva, Merck Serono, Sanofi Genzyme, Novartis, Bayer and Biogen. Christian Eienbroeker: received honoraria for lecturing from Bayer, Biogen, and CSL Behring. Björn Tackenberg: received personal speaker honoraria and consultancy fees as a speaker and advisor from Bayer Healthcare, Biogen, CSL Behring, GRIFOLS, Merck Serono, Novartis, Octapharma, Roche, Sanofi Genzyme, TEVA and UCB Pharma. Volker Limmroth: received honoraria as speaker, AD-Board member or research support from Antisense, Bayer, Biogen, Boehringer, Sanofi Genzyme, Novartis, Pfizer, Roche and Teva. Brigitte Wildemann: received grants and personal fees from Biogen, Merck Serono, Sanofi Genzyme, Novartis and Teva, and personal fees from Bayer Healthcare. Jürgen Haas: declares no conflict of interest. Luisa Klotz: received compensation for serving on scientific advisory boards from Sanofi Genzyme and Novartis, honoraria for lecturing and travel reimbursements from CSL Behring, Merck Serono, Biogen, Sanofi Genzyme, Novartis, and research support from Biogen and Novartis. Heinz Wiendl: received compensation for serving on Scientific Advisory Boards/Steering Committees from Bayer, Biogen, Sanofi Genzyme, Merck Serono and Novartis, honoraria for lecturing and travel reimbursements Bayer, Biogen, CSL Behring, EMD Serono, Fresenius Medical Care, Sanofi Genzyme, Merck Serono, Omniamed, Novartis and Sanofi Aventis. He received compensation as a consultant from Biogen, Merck Serono, Novartis, Roche and Sanofi Genzyme and research support from Bayer, Biogen, Merck Serono, Novartis, Sanofi Genzyme and Teva. Tobias Ruck: received travel reimbursements from Merck Serono and financial research support from Sanofi Genzyme and Novartis and honoraria for lecturing from Sanofi Genzyme, Roche, Biogen, Merck Serono and Teva. Sven G. Meuth: received honoraria for lecturing, travel reimbursements and financial research support from Bayer, Biogen, Sanofi Genzyme, Merck Serono, Merck Sharp & Dohme, Novartis, Novo Nordisk, Sanofi Aventis, UCB and Teva. The submitting author hereby declares that he takes responsibility for conduction and analysis of the data and that he had full access to all study data. The submitting author furthermore declares that there are no competing interests concerning these data and that the authors have all rights to publish the data. The submitted manuscript does not contain data that have been published in any other journal. The authors have no related articles under submission.
Ethical standards
The local institutional review board (IRB) has approved the conduction of this trial. Further details are listed in the “Methods” chapter.
Rights and permissions
About this article
Cite this article
Pfeuffer, S., Schmidt, R., Straeten, F.A. et al. Efficacy and safety of alemtuzumab versus fingolimod in RRMS after natalizumab cessation. J Neurol 266, 165–173 (2019). https://doi.org/10.1007/s00415-018-9117-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-018-9117-z