Abstract
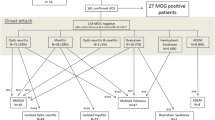
Antibodies against the myelin oligodendrocyte glycoprotein (MOG-Ab) can be detected in various pediatric acquired demyelinating syndromes (ADS). Here, we analyze the spectrum of neuroradiologic findings in children with MOG-Ab and a first demyelinating event. The cerebral and spinal MRI of 69 children with different ADS was assessed in regard to the distribution and characteristics of lesions. Children with acute disseminated encephalomyelitis (n = 36) or neuromyelitis optica spectrum disorder (n = 5) presented an imaging pattern characterized predominantly by poorly demarcated lesions with a wide supra- and infratentorial distribution. Younger children also tended to have poorly defined and widespread lesions. The majority of patients with an isolated optic neuritis (n = 16) only presented small non-specific brain lesions or none at all. A longitudinally extensive transverse myelitis mainly affecting the cervical, and less often so the thoracic, lumbar, and conus regions, was detected in 31 children. The three children of our cohort who were then finally diagnosed with multiple sclerosis had at onset already demarcated white matter lesions as well as transverse myelitis. In conclusion, children with MOG seropositive ADS present disparate, yet characteristic imaging patterns. These patterns have been seen to correlate to the disease entity as well as to age of symptom onset.


Similar content being viewed by others
References
Baumann M, Sahin K, Lechner C, Hennes EM, Schanda K, Mader S, Karenfort M, Selch C, Häusler M, Eisenkölbl A, Salandin M, Gruber-Sedlmayr U, Blaschek A, Kraus V, Leiz S, Finsterwalder J, Gotwald T, Kuchukhidze G, Berger T, Reindl M, Rostásy K (2015) Clinical and neuroradiological differences of paediatric acute disseminating encephalomyelitis with and without antibodies to the myelin oligodendrocyte glycoprotein. J Neurol Neurosurg Psychiatry 86:265–272. https://doi.org/10.1136/jnnp-2014-308346
Lechner C, Baumann M, Hennes EM, Schanda K, Marquard K, Karenfort M, Leiz S, Pohl D, Venkateswaran S, Pritsch M, Koch J, Schimmel M, Häusler M, Klein A, Blaschek A, Thiels C, Lücke T, Gruber-Sedlmayr U, Kornek B, Hahn A, Leypoldt F, Sandrieser T, Gallwitz H, Stoffels J, Korenke C, Reindl M, Rostásy K (2016) Antibodies to MOG and AQP4 in children with neuromyelitis optica and limited forms of the disease. J Neurol Neurosurg Psychiatry 87:897–905. https://doi.org/10.1136/jnnp-2015-311743
Huppke P, Rostasy K, Karenfort M, Huppke B, Seidl R, Leiz S, Reindl M, Gärtner J (2013) Acute disseminated encephalomyelitis followed by recurrent or monophasic optic neuritis in pediatric patients. Mult Scler 19:941–946. https://doi.org/10.1177/1352458512466317
Baumann M, Hennes EM, Schanda K, Karenfort M, Kornek B, Seidl R, Diepold K, Lauffer H, Marquardt I, Strautmanis J, Syrbe S, Vieker S, Höftberger R, Reindl M, Rostásy K (2016) Children with multiphasic disseminated encephalomyelitis and antibodies to the myelin oligodendrocyte glycoprotein (MOG): extending the spectrum of MOG antibody positive diseases. Mult Scler 22:1821–1829. https://doi.org/10.1177/1352458516631038
Rostasy K, Mader S, Schanda K, Huppke P, Gärtner J, Kraus V, Karenfort M, Tibussek D, Blaschek A, Bajer-Kornek B, Leitz S, Schimmel M, Di Pauli F, Berger T, Reindl M (2012) Anti-myelin oligodendrocyte glycoprotein antibodies in pediatric patients with optic neuritis. Arch Neurol 69:752–756. https://doi.org/10.1001/archneurol.2011.2956
Rostásy K, Mader S, Hennes EM, Schanda K, Gredler V, Guenther A, Blaschek A, Korenke C, Pritsch M, Pohl D, Maier O, Kuchukhidze G, Brunner-Krainz M, Berger T, Reindl M (2013) Persisting myelin oligodendrocyte glycoprotein antibodies in aquaporin-4 antibody negative pediatric neuromyelitis optica. Mult Scler 19:1052–1059. https://doi.org/10.1177/1352458512470310
Fernandez-Carbonell C, Vargas-Lowy D, Musallam A, Healy B, McLaughlin K, Wucherpfennig KW, Chitnis T (2016) Clinical and MRI phenotype of children with MOG antibodies. Mult Scler 22:174–184. https://doi.org/10.1177/1352458515587751
Thulasirajah S, Pohl D, Davila-Acosta J, Venkateswaran S (2016) Myelin oligodendrocyte glycoprotein-associated pediatric central nervous system demyelination: clinical course, neuroimaging findings, and response to therapy. Neuropediatrics 47:245–252. https://doi.org/10.1055/s-0036-1583184
Hino-Fukuyo N, Haginoya K, Nakashima I, Sato DK, Takahashi T, Misu T, Fujihara K, Hirose M, Kakisaka Y, Uematsu M, Kobayashi T, Kure S (2015) Clinical features and long-term outcome of a group of Japanese children with inflammatory central nervous system disorders and seropositivity to myelin-oligodendrocyte glycoprotein antibodies. Brain Dev 37:849–852. https://doi.org/10.1016/j.braindev.2015.02.006
Hacohen Y, Absoud M, Deiva K, Hemingway C, Nytrova P, Woodhall M, Palace J, Wassmer E, Tardieu M, Vincent A, Lim M, Waters P (2015) Myelin oligodendrocyte glycoprotein antibodies are associated with a non-MS course in children. Neurol Neuroimmunol Neuroinflamm 2:e81. https://doi.org/10.1212/NXI.0000000000000081
Ketelslegers IA, Van Pelt DE, Bryde S, Neuteboom RF, Catsman-Berrevoets CE, Hamann D, Hintzen RQ (2015) Anti-MOG antibodies plead against MS diagnosis in an acquired demyelinating syndromes cohort. Mult Scler 21:1513–1520. https://doi.org/10.1177/1352458514566666
Hennes EM, Baumann M, Schanda K, Anlar B, Bajer-Kornek B, Blaschek A, Brantner-Inthaler S, Diepold K, Eisenkölbl A, Gotwald T, Kuchukhidze G, Gruber-Sedlmayr U, Häusler M, Höftberger R, Karenfort M, Klein A, Koch J, Kraus V, Lechner C, Leiz S, Leypoldt F, Mader S, Marquard K, Poggenburg I, Pohl D, Pritsch M, Raucherzauner M, Schimmel M, Thiels C, Tibussek D, Vieker S, Zeches C, Berger T, Reindl M, Rostásy K, BIOMARKER Study Group (2017) Prognostic relevance of MOG antibodies in children with an acquired demyelinating syndrome. Neurology 89:900–908. https://doi.org/10.1212/wnl.0000000000004312
Hacohen Y, Mankad K, Chong WK, Barkhof F, Vincent A, Lim M, Wassmer E, Ciccarelli O, Hemingway C (2017) Diagnostic algorithm for relapsing acquired demyelinating syndromes in children. Neurology 89:269–278. https://doi.org/10.1212/WNL.0000000000004117
Jarius S, Ruprecht K, Kleiter I, Borisow N, Asgari N, Pitarokoili K, Pache F, Stich O, Beume LA, Hümmert MW, Ringelstein M, Trebst C, Winkelmann A, Schwarz A, Buttmann M, Zimmermann H, Kuchling J, Franciotta D, Capobianco M, Siebert E, Lukas C, Korporal-Kuhnke M, Haas J, Fechner K, Brandt AU, Schanda K, Aktas O, Paul F, Reindl M, Wildemann B (2016) MOG-IgG in NMO and related disorders: a multicenter study of 50 patients. Part 2: Epidemiology, clinical presentation, radiological and laboratory features, treatment responses, and long-term outcome. J Neuroinflamm 13:280. https://doi.org/10.1186/s12974-016-0718-0
Krupp LB, Tardieu M, Amato MP, Banwell B, Chitnis T, Dale RC, Ghezzi A, Hintzen R, Kornberg A, Pohl D, Rostasy K, Tenembaum S, Wassmer E, International Pediatric Multiple Sclerosis Study Group (2013) International Pediatric Multiple Sclerosis Study Group criteria for pediatric multiple sclerosis and immune-mediated central nervous system demyelinating disorders: revisions to the 2007 definitions. Mult Scler 19:1261–1267. https://doi.org/10.1177/1352458513484547
Mader S, Gredler V, Schanda K, Rostasy K, Dujmovic I, Pfaller K, Lutterotti A, Jarius S, Di Pauli F, Kuenz B, Ehling R, Hegen H, Deisenhammer F, Aboul-Enein F, Storch MK, Koson P, Drulovic J, Kristoferitsch W, Berger T, Reindl M (2011) Complement activating antibodies to myelin oligodendrocyte glycoprotein in neuromyelitis optica and related disorders. J Neuroinflamm 8:184. https://doi.org/10.1186/1742-2094-8-184
Verhey LH, Branson HM, Laughlin S, Shroff MM, Benseler SM, Feldman BM, Streiner DL, Sled JG, Banwell B (2013) Development of a standardized MRI scoring tool for CNS demyelination in children. AJNR Am J Neuroradiol 34:1271–1277. https://doi.org/10.3174/ajnr.A3382
Polman CH, Reingold SC, Banwell B, Clanet M, Cohen JA, Filippi M, Fujihara K, Havrdova E, Hutchinson M, Kappos L, Lublin FD, Montalban X, O’Connor P, Sandberg-Wollheim M, Thompson AJ, Waubant E, Weinshenker B, Wolinsky JS (2011) Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol 69:292–302. https://doi.org/10.1002/ana.22366
Slavin AJ, Johns TG, Orian JM, Bernard CC (1997) Regulation of myelin oligodendrocyte glycoprotein in different species throughout development. Dev Neurosci 19:69–78
Mukherjee P, Miller JH, Shimony JS, Philip JV, Nehra D, Snyder AZ, Conturo TE, Neil JJ, McKinstry RC (2002) Diffusion-tensor MR imaging of gray and white matter development during normal human brain maturation. AJNR Am J Neuroradiol 23:1445–1456
Ben Bashat D, Ben Sira L, Graif M, Pianka P, Hendler T, Cohen Y, Assaf Y (2005) Normal white matter development from infancy to adulthood: comparing diffusion tensor and high b value diffusion weighted MR images. J Magn Reson Imaging 21:503–511
Longoni G, Brown RA, Momayyez Siahkal P, Elliott C, Narayanan S, Bar-Or A, Marrie RA, Yeh EA, Filippi M, Banwell B, Arnold DL, Network Canadian Pediatric Demyelinating Disease (2017) White matter changes in paediatric multiple sclerosis and monophasic demyelinating disorders. Brain 140:1300–1315
Cobo-Calvo Á, Ruiz A, D’Indy H, Poulat AL, Carneiro M, Philippe N, Durand-Dubief F, Deiva K, Vukusic S, Desportes V, Marignier R (2017) MOG antibody-related disorders: common features and uncommon presentations. J Neurol. https://doi.org/10.1007/s00415-017-8583-z
Jurynczyk M, Geraldes R, Probert F, Woodhall MR, Waters P, Tackley G, DeLuca G, Chandratre S, Leite MI, Vincent A, Palace J (2017) Distinct brain imaging characteristics of autoantibody-mediated CNS conditions and multiple sclerosis. Brain 140:617–627. https://doi.org/10.1093/brain/aww350
Juryńczyk M, Tackley G, Kong Y, Geraldes R, Matthews L, Woodhall M, Waters P, Kuker W, Craner M, Weir A, DeLuca GC, Kremer S, Leite MI, Vincent A, Jacob A, de Sèze J, Palace J (2017) Brain lesion distribution criteria distinguish MS from AQP4-antibody NMOSD and MOG-antibody disease. J Neurol Neurosurg Psychiatry 88:132–136. https://doi.org/10.1136/jnnp-2016-314005
Zuccoli G, Panigrahy A, Sreedher G, Bailey A, Laney EJ 4th, La Colla L, Alper G (2014) Vasogenic edema characterizes pediatric acute disseminated encephalomyelitis. Neuroradiology 56:679–684. https://doi.org/10.1007/s00234-014-1379-2
Wong YY, van Pelt ED, Ketelslegers IA, Catsman-Berrevoets CE, Hintzen RQ, Neuteboom RF, Dutch Study Group for Paediatric Multiple Sclerosis and Acute Disseminated Encephalomyelitis (2017) Evolution of MRI abnormalities in paediatric acute disseminated encephalomyelitis. Eur J Paediatr Neurol 21:300–330. https://doi.org/10.1016/j.ejpn.2016.08.014
Acknowledgements
This work was supported by a grant from the Anniversary Fund of the Austrian National Bank to KR (Grant number 14158); and the research grant “BIG WIG MS” from the Austrian Federal Ministry of Science, Research and Economy to MR.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Ethical standards
Approval for this study was obtained from the institution’s review board. All included patients or caregivers provided written informed consent.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Baumann, M., Grams, A., Djurdjevic, T. et al. MRI of the first event in pediatric acquired demyelinating syndromes with antibodies to myelin oligodendrocyte glycoprotein. J Neurol 265, 845–855 (2018). https://doi.org/10.1007/s00415-018-8781-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-018-8781-3