Abstract
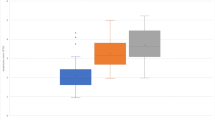
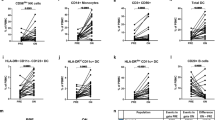
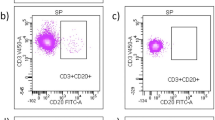
Response to interferon-beta (IFN-beta) treatment is heterogeneous in multiple sclerosis (MS). We aimed to search for biomarkers predicting no evidence of disease activity (NEDA) status upon IFN-beta treatment in MS. 119 patients with relapsing–remitting MS (RRMS) initiating IFN-beta treatment were included in the study, and followed prospectively for 2 years. Neutralizing antibodies (NAb) were explored in serum samples obtained after 6 and 12 months of IFN-beta treatment. Soluble cytokines and blood lymphocytes were studied in basal samples by ELISA and flow cytometry, respectively. 9% of patients developed NAb. These antibodies were more frequent in patients receiving IFN-beta 1b than in those treated subcutaneous (p = 0.008) or intramuscular (p < 0.0001) IFN-beta 1a. No patient showing NAb remained NEDA during follow-up. Basal immunological variables are also associated with patient response. Percentages below 3% of CD19 + CD5 + cells (AUC 0.74, CI 0.63–0.84; OR 10.68, CI 3.55–32.15, p < 0.0001; Likelihood ratio 4.28) or above 2.6% of CD8 + perforin + T cells (AUC 0.79, CI 0.63–0.96; OR 6.11, CI 2.0–18.6, p = 0.0009; Likelihood ratio 5.47) increased the probability of achieving NEDA status during treatment. Basal blood immune cell subsets contribute to identify MS patients with a high probability of showing an optimal response to IFN-beta.



Similar content being viewed by others
Change history
24 November 2017
The author claims that his name is incorrectly listed on PubMed. It seems that the first and last name has been mixed up.
References
The IFNB Multiple Sclerosis Study Group (1993) Interferon beta-1b is effective in relapsing-remitting multiple sclerosis. I. Clinical results of a multicenter, randomized, double-blind, placebo-controlled trial. Neurology 43:655–661
Jacobs LD, Cookfair DL, Rudick RA et al (1996) Intramuscular interferon beta-1a for disease progression in relapsing multiple sclerosis. The Multiple Sclerosis Collaborative Research Group (MSCRG). Ann Neurol 39:285–294
PRISMS (Prevention of Relapses and Disability by Interferon beta-1a Subcutaneously in Multiple Sclerosis) (1998) Study group randomised double-blind placebo-controlled study of interferon beta-1a in relapsing/remitting multiple sclerosis. Lancet 352:1498–1504
Einarson TR, Bereza BG, Machado M (2017) Comparative effectiveness of interferons in relapsing–remitting multiple sclerosis: a meta-analysis of real-world studies. Curr Med Res Opin 33:579–593
Giovannoni G, Turner B, Gnanapavan S, Offiah C, Schmierer K, Marta M (2015) Is it time to target no evident disease activity (NEDA) in multiple sclerosis? Mult Scler Relat Disord 4:329–333
Rotstein DL, Healy BC, Malik MT, Chitnis T, Weiner HL (2015) Evaluation of no evidence of disease activity in a 7-year longitudinal multiple sclerosis cohort. JAMA Neurol 72:152–158
Río J, Castilló J, Rovira A et al (2009) Measures in the first year of therapy predict the response to interferon beta in MS. Mult Scler 15:848–853
Martínez-Rodríguez JE, López-Botet M, Munteis E, Rio J, Roquer J, Montalban X, Comabella M (2011) Natural killer cell phenotype and clinical response to interferon-beta therapy in multiple sclerosis. Clin Immunol 141:348–356
Hartung HP, Steinman L, Goodin DS et al (2013) Interleukin 17F level and interferon beta response in patients with multiple sclerosis. JAMA Neurol 70:1017–1021
Pachner AR, Dail D, Pak E, Narayan K (2005) The importance of measuring IFNbeta bioactivity: monitoring in MS patients and the effect of anti-IFNbeta antibodies. J Neuroimmunol 166:180–188
Ransohoff RM, Hafler DA, Lucchinetti CE (2015) Multiple sclerosis—a quiet revolution. Nat Rev Neurol 11:134–142
De Mercanti S, Rolla S, Cucci A et al (2016) Alemtuzumab long-term immunologic effect: Treg suppressor function increases up to 24 months. Neurol Neuroimmunol Neuroinflamm 3:e194
Blumenfeld S, Staun-Ram E, Miller A (2016) Fingolimod therapy modulates circulating B cell composition, increases B regulatory subsets and production of IL-10 and TGFβ in patients with multiple sclerosis. J Autoimmun 70:40–51
Bielekova B, Catalfamo M, Reichert-Scrivner S et al (2006) Regulatory CD56 (bright) natural killer cells mediate immunomodulatory effects of IL-2R alpha-targeted therapy (daclizumab) in multiple sclerosis. Proc Natl Acad Sci USA 103:5941–5946
Polman CH, Reingold SC, Banwell B et al (2011) Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol 69:292–302
García-Montojo M, Domínguez-Mozo MI, De las Heras V, Bartolome M, Garcia-Martinez A, Arroyo R, Alvarez-Lafuente R (2010) Neutralizing antibodies, MxA expression and MMP-9/TIMP-1 ratio as markers of bioavailability of interferon beta treatment in multiple sclerosis patients. A two years follow-up study. Eur J Neurol 17:470–478
Kawade Y (1986) Quantitation of neutralization of interferon by antibody. Methods Enzymol 119:558–573
Reimann KA, Chernoff M, Wilkening CL, Nickerson CE, Landay AL (2000) Preservation of lymphocyte immunophenotype and proliferative responses in cryopreserved peripheral blood mononuclear cells from human immunodeficiency virus type 1-infected donors: implications for multicenter clinical trials. The ACTG immunology advanced technology laboratories. Clin Diag Lab Immunol 7:352–359
Bushnell SE, Zhao Z, Stebbins CC et al (2012) Serum IL-17F does not predict poor response to IM IFNbeta-1a in relapsing–remitting MS. Neurology 79:531–537
Bertolotto A, Sala A, Malucchi S, Marnetto F, Caldano M, Di Sapio A, Capobianco M, Gilli F (2004) Biological activity of interferon betas in patients with multiple sclerosis is affected by treatment regimen and neutralising antibodies. J Neurol Neurosurg Psychiatry 75:1294–1299
Bertolotto A, Malucchi S, Sala A et al (2002) Differential effects of three interferon betas on neutralising antibodies in patients with multiple sclerosis: a follow up study in an independent laboratory. J Neurol Neurosurg Psychiatry 73:148–153
Frisullo G, Nociti V, Iorio R et al (2009) Regulatory T cells fail to suppress CD4T + -bet + T cells in relapsing multiple sclerosis patients. Immunology 127:418–428
Seidi OA, Semra YK, Sharief MK (2002) Expression of CD5 on B lymphocytes correlates with disease activity in patients with multiple sclerosis. J Neuroimmunol 133:205–210
Villar LM, Espiño M, Roldán E, Marín N, Costa-Frossard L, Muriel A, Alvarez-Cermeño JC (2011) Increased peripheral blood CD5 + B cells predict earlier conversion to MS in high-risk clinically isolated syndromes. Mult Scler 17:690–694
Praksova P, Stourac P, Bednarik J, Vlckova E, Mikulkova Z, Michalek J (2012) Immunoregulatory T cells in multiple sclerosis and the effect of interferon beta and glatiramer acetate treatment on T cell subpopulations. J Neurol Sci 319:18–23
Skulina C, Schmidt S, Dornmair K et al (2004) Multiple sclerosis: brain-infiltrating CD8 + T cells persist as clonal expansions in the cerebrospinal fluid and blood. Proc Natl Acad Sci USA 101:2428–2433
Salou M, García A, Michel L et al (2015) Expanded CD8 T-cell sharing between periphery and CNS in multiple sclerosis. Ann Clin Transl Neurol 2:609–622
Acknowledgements
This work was supported by Grants PI15/00513, RD16/0015/0001, RD16/0015/0004 and RD16/0015/0013 from the Fondo para la Investigación Sanitaria, Instituto de Salud Carlos III, Ministerio de Economía y Competitividad, Spain and FEDER.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical statement
The study protocol was approved by the Ethics Committee of Hospital Universitario Ramón y Cajal (Madrid, Spain) and of Hospital Universitari Vall d’Hebron (Barcelona, Spain). The study was carried out according to the International Conference on Harmonization Guidelines for Good Clinical Practice and the Declaration of Helsinki.
Informed consent
Every patient provided written informed consent before entering the study.
Conflicts of interest
LMV, LCF, SSM, JCA-C, JR and XM received payment for lecturing or travel expenses or research Grants or consultancy from Merck-Serono, Biogen, Sanofi-Genzyme, Roche, Bayer and Novartis. The remaining authors declare no conflicts of interest.
Additional information
José C. Álvarez-Cermeño and Luisa M. Villar were principal co-investigators.
A correction to this article is available online at https://doi.org/10.1007/s00415-017-8679-5.
Rights and permissions
About this article
Cite this article
Alenda, R., Costa-Frossard, L., Alvarez-Lafuente, R. et al. Blood lymphocyte subsets identify optimal responders to IFN-beta in MS. J Neurol 265, 24–31 (2018). https://doi.org/10.1007/s00415-017-8625-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-017-8625-6