Abstract
The autosomal recessive spinocerebellar ataxias are an exciting field of study, with a growing number of causal genes and an expanding phenotypic spectrum. SYNE1 was originally discovered in 2007 as the causal gene underlying autosomal recessive spinocerebellar ataxia 1, a disease clinically thought to manifest with mainly pure cerebellar ataxia. Since the original report SYNE1 mutations have also been identified in families with motor neuronopathy and arthrogryposis but few families have been screened as the gene is very large at 146 exons in length. We screened 196 recessive and sporadic ataxia patients for mutations in SYNE1 using next generation sequencing in order to assess its frequency and extend the clinicogenetic spectrum. We identified four novel truncating mutations spread throughout the SYNE1 gene from three families living in London that originated from England, Turkey and Sri Lanka. The phenotype was mainly pure cerebellar ataxia in two families, cognitive decline was present in all three families, axonal neuropathy in one family and marked spasticity in the Turkish family, with a range of disease severities. Searching for genotype–phenotype correlations in the SYNE1 gene, defects located near the 3′ prime end of the gene are more frequently associated with motor neuron or neuromuscular involvement so far. Our data indicate SYNE1 mutations are not an uncommon cause of recessive ataxia with or without additional clinical features in patients from various ethnicities. The use of next generation sequencing allows the rapid analysis of large genes and will likely reveal more SYNE1 associated cases and further expand genotype–phenotype correlations.
Similar content being viewed by others
Introduction
The recessive spinocerebellar ataxias (ARCAs or SCARs) are a complex group of neurodegenerative conditions with significant genetic and clinical heterogeneity [1]. They are usually characterized by early-onset ataxia with a variable range of other neurological manifestations such as pyramidal signs, endocrine manifestations, epilepsy, cognitive deterioration and peripheral neuropathy [2, 3]. To date, there are over 70 genes that can cause recessive ataxia and this includes 21 SCAR genes [4]. In 2007, SYNE1 was identified as a cause of pure cerebellar phenotypes. It was termed recessive ataxia of Beauce (SCAR8 or ARCA1, MIM# 610743), since it was originally identified in a number of French-Canadian families originating from the Beauce and Bas-St-Laurent regions of Quebec [5].
Subsequent investigations have identified more than 11 biallelic truncating mutations and one homozygous missense mutation in individuals originating from Canada, France, Brazil and Japan with pure cerebellar ataxia [6–9]. More recently families from Japan and Turkey with homozygous truncating SYNE1 mutations have been associated with a motor neuron phenotype in addition to cerebellar ataxia [7, 10]. Furthermore, heterozygous missense mutations in SYNE1 have been identified in dominant muscular dystrophy and two unrelated probands with Emery-Dreifuss muscular dystrophy (MIM# 612998) where only the exons contributing to the muscle specific isoform of SYNE1 were investigated [11, 12]. Besides, homozygous acceptor splice site mutations two basepairs 5-prime to exon 137 were identified in a consanguineous Palestinian pedigree with myogenic arthrogryposis [13]. In addition defects in SYNE1 have been shown to be increased in exome sequencing studies of mental retardation and autism [14, 15].
SYNE1 encodes the spectrin repeat-containing nuclear envelope protein 1, a structural protein expressed in various tissues and believed to link the nucleoskeleton to the inner and outer nuclear membrane, to membranes of cell organelles, to the actin cytoskeleton, and to the sarcomere in muscle [11, 16, 17]. Data from mice suggest a critical role in neurogenesis and neuronal migration for SYNE1 as an organiser of nucleokinesis in the interplay with other complex proteins such as SYNE2, SUN1 and SUN2 [18]. However, its direct functional role in the human central nervous system, and particularly in the cerebellum, remains understudied.
In this study, we examined an ethnically diverse UK cohort of autosomal recessive families and sporadic cerebellar ataxia patients through a combination of targeted next generation sequencing and exome sequencing, identifying biallelic SYNE1 mutations in three families from England, Turkey and Sri Lanka with the phenotypes and severities described here.
Methods
Patients
All patients were recruited through the Neurogenetics service at the National Hospital for Neurology and Neurosurgery, Queen Square, London and gave informed consent. All patients had a diagnosis of progressive cerebellar ataxia with either known autosomal recessive or presumed sporadic inheritance with early-onset disease. In total 196 patients were screened for SYNE1 mutations through either exome sequencing (110 patients) or targeted next generation sequencing (86 patients).
Genetic analysis
DNA was extracted from peripheral leucocytes of all patients in the diagnostic lab, using standard procedures. Additional samples were taken from affected or unaffected relatives to test for mutation segregation where appropriate [19]. Exome sequencing libraries were prepared using Illumina Nextera Rapid Capture Exome Kits following the manufacturer’s recommendation. Libraries were indexed and sequenced on an Illumina HiSeq2500 machine. A custom sequencing panel was designed to amplify the coding exons of SYNE1 using the Illumina Truseq Custom Amplicon v1.5 Kits. Libraries were prepared in keeping with the standard recommended protocol and then sequenced on an Illumina MiSeq machine. Bioinformatic analysis was the same for both exome sequencing and targeted next generation sequencing: Reads were aligned to the hg19 genome build using Novoalign with variant calling performed using SAMtools and Genome Analysis Toolkit Best Practices (GATK, Broad Institute). Variant annotation was achieved with ANNOVAR and coverage metrics were investigated using a modified in-house Bedtools coverageBed script. All SYNE1 annotations and mutation locations given below are for the refseq NM_033071 transcript (ENST00000423061).
The final list of called variants in SYNE1 was filtered according to the following criteria:
(1) nonsynonymous variants present in a homozygous or compound heterozygous state only, (2) Quality >30, (3) depth >10 and (4) minor allele frequency in exome variant server, ExAC and 1000 g <0.005.
Identified variants were confirmed using Sanger sequencing (see Supplementary Table 1 for primers employed) in affected cases and in parents or unaffected siblings where available to confirm segregation or mutation phase for compound heterozygous mutations.
Results
Family I—Patient I:1 and I:4 are siblings from a non-consanguineous English family. The two siblings were noted to be poor at sports in school and had poor handwriting. There was, however, no concern about gait or balance until much later in life. Patient I:1 sustained a significant head injury at the age of 21 and was noted to have slightly slurred speech after his accident which was attributed at the time to multiple jaw fractures. There was no perceptible change in gait or coordination until the age of 40 when he noticed difficulty rising from a chair and sustained several falls when walking. He is currently 65 years of age and now has significant gait ataxia and dysarthria. His 49-year-old sister (I:4) did not notice any deterioration in her gait until age 32 after she also was involved in a road traffic accident. She has noticed a slowly progressive deterioration in her gait and speech since this time. Examination of both siblings was very similar with both exhibiting clinical signs of cerebellar ataxia with broken ocular pursuit, cerebellar dysarthria and limb and gait ataxia. Reflexes were normal and there was no clinical and electrophysiologic evidence of neuropathy. Investigations showed both had cerebellar atrophy on MRI (see Fig. 1b for patient I:4).
Cognitive profile of the proband I:4 was as follows: WAIS-III showed verbal IQ of 70 and performance IQ of 67. Verbal subtest scores were impaired (Arithmetic, Digit Span), borderline impaired (Similarities) and low average (Vocabulary). Performance subtest scores were impaired (Block Design) and borderline impaired (Picture Completion, Picture Arrangement). Thus, current performance on the WAIS-III reflects cognitive under-functioning. Overall, memory skills were satisfactory. Thus, performance on a test of verbal recognition memory was good (Recognition Memory Test for Words: 95th percentile). Performance on a test of visual recall memory was good (AMIPB Figure, immediate: 25–50th percentile, and delayed: 50–75th percentile), and performance on a verbal learning task was satisfactory (AMIPB List Learning, A1–A5: 25–50th percentile, A6: 25th percentile). In contrast, performance on a test of visual recognition memory was poor (recognition memory test for faces: 10th percentile). Nominal skills are adequate (graded naming test: 10–25th percentile), and arithmetic skills were impaired (graded difficulty arithmetic test).
Visuospatial skills were weak (VOSP Position Discrimination: <5 % cut-off), and her copy of a complex figure was poor (AMIPB Figure). In contrast, visuoperceptual skills were satisfactory (VOSP incomplete letters: >5 % cut-off). There was evidence of mild executive dysfunction. Thus, phonemic verbal fluency was a little reduced (‘S’: 10), her performance on the Stroop task was impaired (<2nd percentile) and on the Hayling Sentence Completion Task her performance was poor. In contrast, she was able to identify 6/6 categories on the Modified Card Sorting Test, albeit with a little prompting. Speed of information processing was within normal limits (oral symbol digit modalities test). Performance was abnormal on a test of sustained attention (TEA, Elevator Counting: 5/7) and in the borderline impaired range on a test of selective attention (TEA, Elevator Counting with Distraction: 5–10th percentile).
In summary, the cognitive scores of the proband I:4 reflect cognitive under-functioning. On focal cognitive tests, the main findings are weak arithmetic skills, poor visuospatial skills, evidence of executive dysfunction and reduced speed of information processing. Performance on tests of memory and naming were satisfactory, and visuoperceptual skills were intact.
In both affected siblings, two novel compound heterozygous truncating mutations were identified in exon 18 (c.1849G>T:p.E617X) and exon 99 (c.18431G>A: p.W6144X) of SYNE1. One of the two unaffected siblings was available for testing and carried only the exon 98 mutation confirming the mutation phase to be trans (see Fig. 1c).
Family II—Patient II:1 is one of 11 siblings from consanguineous parents (1st cousins) of Turkish origin. She was completely well until age 18 years when she developed progressive gait ataxia and dysarthria. At age 23 she was still mobilising without aid but had a high frequency of falls and required adaptations to her home to ensure safety. The patient is now 34 years old. She has 4 affected siblings who all developed symptoms with onset in their late teens and were reported to be very similar to the index case although unfortunately neither clinical notes, nor DNA were available as none of the other family members are resident in the UK and contact has been interrupted. Clinical examination of the index patient showed cognitive difficulties at the bedside but she was not formally assessed. There was broken pursuit eye movements, mild to moderate finger nose ataxia and marked gait ataxia. Reflexes were brisk in the lower limbs with sustained clonus at both ankles and extensor plantar reflexes.
Brain MRI of the index case showed marked cerebellar atrophy. Nerve conduction studies showed no evidence of peripheral neuropathy. A novel homozygous variant in exon 108 of SYNE1 (c.19897C>T p.Q6633X) was identified in the proband (see Fig. 2a, left panel). DNA for the parents and siblings was not available.
Family III—The proband (III:1) is a 38-year-old Sri Lankan man who originally developed gait and balance problems at 22 years. He is the second of five siblings that are all well and without neurological problems. The patient’s parents were not known to be related. He was fit and active in sports as a child and has despite the obvious gait difficulties remained independent without usage of walking aids till present. Clinical examination showed hypermetric saccades, limb ataxia and extensor plantar reflexes although reflexes were reduced. MRI brain is shown above with cerebellar atrophy (Fig. 2b) and nerve conduction studies showed an axonal neuropathy, other screening blood tests were normal. The examination of this patient is shown on the attached video (see Supplementary Video 1).
Cognitive profile in the proband was as follows: WAIS-III showed a verbal IQ of 76 and performance IQ of 78. Verbal subtest scores were average (Arithmetic) low average (Digit Span), and borderline impaired (Similarities and impaired Vocabulary). Performance subtest scores were low average (Block Design, Picture Arrangement) and borderline impaired (Picture Completion). Performance was normal on a test of verbal memory (short Recognition Memory Test for Words: 75th percentile). In contrast, his scores were poor on two tests of visual memory (Recognition Memory Test for Faces: <10th percentile; AMIPB Figure Recall <5th percentile). Naming skills were satisfactory when considering language and cultural factors. He was able to name 16/30 items on the Oldfield Naming Test. Performance was normal on tests of visual perception and visual spatial functioning (Incomplete Letters: >5 % cut-off, Position Discrimination: >5 % cut-off). Performance was satisfactory on a test of Arithmetic (Graded Difficulty Arithmetic Test: 10–25th percentile). Performance on the simple Stroop test was satisfactory and he passed the Weigl Sorting test. Processing speed is reduced (Symbol Digit Modalities Test <5th percentile). His performance was poor on a test of selective attention (Elevator Counting with Distraction).
In summary, the cognitive scores were mildly impaired on both the verbal and performance scales of the WAIS-III. On a series of focal cognitive tests he presented with a poor performance on some tests of visual memory. In addition, processing speed was reduced and performance was poor on a test of selective attention.
A novel, truncating homozygous variant in SYNE1, exon 77 c.13429C>T:p.Q4477X was identified (See Fig. 2a, right panel).
Discussion
To date, homozygous loss-of-function mutations in SYNE1 have been reported in French-Canadian, French, Japanese, Turkish and Brazilian individuals [6, 7, 10]. With the first British and Sri Lankan cases detected in this study, we further extend the ethnic diversity underlying SYNE1 associated cerebellar ataxia. A summary of pheno- and genotype of these and all other recently reported SYNE1 mutations can be seen in Table 1.
All novel cases reported in our study are associated with truncating mutations likely to result in production of a truncated protein and hence a complete loss or severe reduction of protein function. Clinically, there was a range in the disease age at onset and a possible negative correlation between the position of the stop codon in the reading frame and the severity or age at onset in our cases: e.g., our Turkish case has the most 3-prime mutation of all four; however, this patient presented with the earliest age at onset (18 years) and additional pyramidal manifestations absent in the other 3 cases. Additionally, looking at cases I:1 and I:4 from our series, phenotypic heterogeneity can be observed within families. Even though based on very low numbers of patients, these observations are mirrored via the other recent reports in the literature (see Table 1; Fig. 3) with SYNE1 associated cerebellar ataxia being relatively mild and slowly progressive. Our affected cases from families I and III are fairly typical of previously reported cases, although the family from Sri Lanka had an axonal neuropathy. Cognitive decline was evident in all three families, which has been reported in the past in an extensive report on patients from Quebec [20].
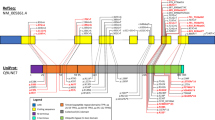
Locations of reported mutations associated with SYNE1 ataxic phenotypes. Mutations in black are from the discovery study, blue mutations are mutations reported since and red are the mutations identified in our study. Yellow filled boxes mark mutations associated with motor neuron phenotypes. Dotted lines connect the further 3-prime mutation with their partner mutation in compound heterozygous cases. Cave: sizes of black bars do not proportionally represent the sizes of the different 146 exons of SYNE1
The proband from family II, however, exhibited marked lower limb pyramidal signs with hyperreflexia and ankle clonus. Of note, there were no variants in any other ataxia or hereditary spastic paraparesis genes identified in the exome sequence data of this individual. Recently, two other Turkish families have been described with affected members manifesting a spastic ataxia and motor neuron phenotype associated with two novel homozygous SYNE1 nonsense mutations (R7842X and Q7644X) [10] (also see Fig. 3 for all mutations associated with motor neuron disease as represented by yellow filled boxes).
Interestingly, all recent mutations presenting with significant pyramidal signs were located towards the 3′ prime end of the gene and seemed to be homozygous mutations and not compound heterozygous mutations. However, as with the age at onset and disease severity, this observation is interesting, but based on a very low number of cases. Furthermore, one of the homozygous mutations reported in the initial study in 2007 (p.Q7640X) is located far 3′ prime of the gene as well, but has been reported with pure cerebellar ataxia as part of the initial cohort (see Fig. 3). It is highly likely that further genetic, epigenetic and environmental modifiers are contributing to the phenotypic variability and it is recommended therefore that SYNE1 mutations be considered also in the aetiology of complex as well as pure recessive ataxia. Further development and establishment of next generation techniques in clinical diagnostics will reduce costs and involved timespans till diagnosis to enable SYNE1 associated cerebellar ataxia to be more readily identified in the future. However, given the significant size of this gene (146 coding exons, 27,436 base pairs, 8749 amino acids), it is likely to inherently harbour a great deal of genetic variation, and cautious interpretation of identified variants will be needed to correctly infer true pathogenic mutations as opposed to benign polymorphisms.
Conclusions
Here, we expand the ethnic and genetic diversity of SYNE1 associated cerebellar ataxia, an important gene to be screened in recessive and sporadic cases. We demonstrate four novel truncating mutations in SYNE1 in pedigrees from British, Sri Lankan and Turkish origin. We observe a range of inter-family severities, extra-cerebellar and cognitive features and possible genotype–phenotype correlations.
References
Anheim M, Tranchant C, Koenig M (2012) The autosomal recessive cerebellar ataxias. New England J Med 366(7):636–646. doi:10.1056/NEJMra1006610
Sailer A, Scholz SW, Gibbs JR, Tucci A, Johnson JO, Wood NW, Plagnol V, Hummerich H, Ding J, Hernandez D, Hardy J, Federoff HJ, Traynor BJ, Singleton AB, Houlden H (2012) Exome sequencing in an SCA14 family demonstrates its utility in diagnosing heterogeneous diseases. Neurology 79(2):127–131. doi:10.1212/WNL.0b013e31825f048e
Sailer A, Houlden H (2012) Recent advances in the genetics of cerebellar ataxias. Curr Neurol Neurosci Rep 12(3):227–236. doi:10.1007/s11910-012-0267-6
http://neuromuscular.wustl.edu/ataxia/recatax.html (2016) Recessive Ataxia
Gros-Louis F, Dupre N, Dion P, Fox MA, Laurent S, Verreault S, Sanes JR, Bouchard JP, Rouleau GA (2007) Mutations in SYNE1 lead to a newly discovered form of autosomal recessive cerebellar ataxia. Nat Genet 39(1):80–85. doi:10.1038/ng1927
Noreau A, Bourassa CV, Szuto A, Levert A, Dobrzeniecka S, Gauthier J, Forlani S, Durr A, Anheim M, Stevanin G, Brice A, Bouchard JP, Dion PA, Dupre N, Rouleau GA (2013) SYNE1 mutations in autosomal recessive cerebellar ataxia. JAMA Neurol 70(10):1231–1296. doi:10.1001/jamaneurol.2013.3268
Izumi Y, Miyamoto R, Morino H, Yoshizawa A, Nishinaka K, Udaka F, Kameyama M, Maruyama H, Kawakami H (2013) Cerebellar ataxia with SYNE1 mutation accompanying motor neuron disease. Neurology 80(6):600–601. doi:10.1212/WNL.0b013e3182815529
Dupre N, Gros-Louis F, Chrestian N, Verreault S, Brunet D, de Verteuil D, Brais B, Bouchard JP, Rouleau GA (2007) Clinical and genetic study of autosomal recessive cerebellar ataxia type 1. Ann Neurol 62(1):93–98. doi:10.1002/ana.21143
Dupre N, Gros-Louis F, Bouchard JP, Noreau A, Rouleau GA (1993) SYNE1-related autosomal recessive cerebellar ataxia. In: Pagon RA, Adam MP, Ardinger HH et al. (eds) Gene Reviews (R). Seattle
Ozoguz A, Uyan O, Birdal G, Iskender C, Kartal E, Lahut S, Omur O, Agim ZS, Eken AG, Sen NE, Kavak P, Saygi C, Sapp PC, Keagle P, Parman Y, Tan E, Koc F, Deymeer F, Oflazer P, Hanagasi H, Gurvit H, Bilgic B, Durmus H, Ertas M, Kotan D, Akalin MA, Gulluoglu H, Zarifoglu M, Aysal F, Dosoglu N, Bilguvar K, Gunel M, Keskin O, Akgun T, Ozcelik H, Landers JE, Brown RH, Basak AN (2015) The distinct genetic pattern of ALS in Turkey and novel mutations. Neurobiol Aging 36 (4):1764 e1769–e1718. doi:10.1016/j.neurobiolaging.2014.12.032
Zhang Q, Bethmann C, Worth NF, Davies JD, Wasner C, Feuer A, Ragnauth CD, Yi Q, Mellad JA, Warren DT, Wheeler MA, Ellis JA, Skepper JN, Vorgerd M, Schlotter-Weigel B, Weissberg PL, Roberts RG, Wehnert M, Shanahan CM (2007) Nesprin-1 and -2 are involved in the pathogenesis of Emery Dreifuss muscular dystrophy and are critical for nuclear envelope integrity. Hum Mol Genet 16(23):2816–2833. doi:10.1093/hmg/ddm238
Fanin M, Savarese M, Nascimbeni AC, Di Fruscio G, Pastorello E, Tasca E, Trevisan CP, Nigro V, Angelini C (2015) Dominant muscular dystrophy with a novel SYNE1 gene mutation. Muscle Nerve 51(1):145–147. doi:10.1002/mus.24357
Attali R, Warwar N, Israel A, Gurt I, McNally E, Puckelwartz M, Glick B, Nevo Y, Ben-Neriah Z, Melki J (2009) Mutation of SYNE-1, encoding an essential component of the nuclear lamina, is responsible for autosomal recessive arthrogryposis. Hum Mol Genet 18(18):3462–3469. doi:10.1093/hmg/ddp290
Schuurs-Hoeijmakers JH, Vulto-van Silfhout AT, Vissers LE, van de V II, van Bon BW, de Ligt J, Gilissen C, Hehir-Kwa JY, Neveling K, del Rosario M, Hira G, Reitano S, Vitello A, Failla P, Greco D, Fichera M, Galesi O, Kleefstra T, Greally MT, Ockeloen CW, Willemsen MH, Bongers EM, Janssen IM, Pfundt R, Veltman JA, Romano C, Willemsen MA, van Bokhoven H, Brunner HG, de Vries BB, de Brouwer AP (2013) Identification of pathogenic gene variants in small families with intellectually disabled siblings by exome sequencing. J Med Genet 50(12):802–811. doi:10.1136/jmedgenet-2013-101644
Yu TW, Chahrour MH, Coulter ME, Jiralerspong S, Okamura-Ikeda K, Ataman B, Schmitz-Abe K, Harmin DA, Adli M, Malik AN, D’Gama AM, Lim ET, Sanders SJ, Mochida GH, Partlow JN, Sunu CM, Felie JM, Rodriguez J, Nasir RH, Ware J, Joseph RM, Hill RS, Kwan BY, Al-Saffar M, Mukaddes NM, Hashmi A, Balkhy S, Gascon GG, Hisama FM, LeClair E, Poduri A, Oner O, Al-Saad S, Al-Awadi SA, Bastaki L, Ben-Omran T, Teebi AS, Al-Gazali L, Eapen V, Stevens CR, Rappaport L, Gabriel SB, Markianos K, State MW, Greenberg ME, Taniguchi H, Braverman NE, Morrow EM, Walsh CA (2013) Using whole-exome sequencing to identify inherited causes of autism. Neuron 77(2):259–273. doi:10.1016/j.neuron.2012.11.002
Zhang Q, Ragnauth C, Greener MJ, Shanahan CM, Roberts RG (2002) The nesprins are giant actin-binding proteins, orthologous to Drosophila melanogaster muscle protein MSP-300. Genomics 80(5):473–481
Zhang Q, Skepper JN, Yang F, Davies JD, Hegyi L, Roberts RG, Weissberg PL, Ellis JA, Shanahan CM (2001) Nesprins: a novel family of spectrin-repeat-containing proteins that localize to the nuclear membrane in multiple tissues. J Cell Sci 114(Pt 24):4485–4498
Zhang X, Lei K, Yuan X, Wu X, Zhuang Y, Xu T, Xu R, Han M (2009) SUN1/2 and Syne/Nesprin-1/2 complexes connect centrosome to the nucleus during neurogenesis and neuronal migration in mice. Neuron 64(2):173–187. doi:10.1016/j.neuron.2009.08.018
Houlden H, King RH, Hashemi-Nejad A, Wood NW, Mathias CJ, Reilly M, Thomas PK (2001) A novel TRK A (NTRK1) mutation associated with hereditary sensory and autonomic neuropathy type V. Ann Neurol 49(4):521–525
Laforce R Jr, Buteau JP, Bouchard JP, Rouleau GA, Bouchard RW, Dupre N (2010) Cognitive impairment in ARCA-1, a newly discovered pure cerebellar ataxia syndrome. Cerebellum 9(3):443–453. doi:10.1007/s12311-010-0184-7
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author (Henry Houlden) states that there is no conflict of interest of any type.
Ethical standard
The study procedures were approved by the University Health Network Research Ethics Board and were performed in accordance with the ethical principles and guidelines for the protection of human subjects of research.
Additional information
S. Wiethoff and J. Hersheson equal contribution.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Wiethoff, S., Hersheson, J., Bettencourt, C. et al. Heterogeneity in clinical features and disease severity in ataxia-associated SYNE1 mutations. J Neurol 263, 1503–1510 (2016). https://doi.org/10.1007/s00415-016-8148-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-016-8148-6