Abstract
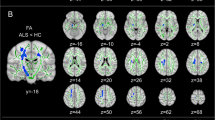
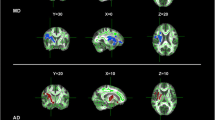
Previous neuroimaging studies have revealed that both gray matter (GM) and white matter (WM) are altered in several morphological aspects in amyotrophic lateral sclerosis (ALS). However, the relations between GM and WM measures and their contributions to clinical features remain in doubt. In this study, we acquired high-resolution diffusion tensor imaging along with structural magnetic resonance imaging data on 20 patients with clinical evidence of ALS and 21 matched healthy controls. WM microstructural metrics and cortical thickness were measured to characterize the whole brain WM and GM degenerative patterns. Probabilistic diffusion tractography was used to reconstruct the tracts from the WM regions characterized by fractional anisotropy (FA) decrease in patients. Decreased FA and increased radial diffusivity was observed in WM regions of the bilateral corticospinal tracts (CST) and callosal motor fibers in the ALS patients, while the superior longitudinal fasciculus exhibited a changing trend. Cortical thinning was found in the anatomically congruent regions, including the motor-related cortices (i.e., bilateral precentral gyri, dorsal premotor cortices, and left supplementary motor area), prefrontal and occipito-parietal regions. However, there was no significant relationship between FA reduction and cortical thinning. Finally, patients with faster clinical progression showed more severe cortical thinning of the left precentral gyrus and FA reduction of the left CST. Together, these findings suggest that ALS is multisystem degeneration involving both the widespread cortices and the underlying WM fibers. GM and WM changes might play distinct roles in the disease progression.



Similar content being viewed by others
Abbreviations
- AD:
-
Axial diffusivity
- ALS:
-
Amyotrophic lateral sclerosis
- ALSFRS-R:
-
ALS Functional Rating Scale-Revised
- CST:
-
Corticospinal tracts
- DTI:
-
Diffusion tensor imaging
- FA:
-
Fractional anisotropy
- FWE:
-
Family-wise error
- GM:
-
Gray matter
- MNI:
-
Montreal Neurological Institute
- MRI:
-
Magnetic resonance imaging
- preCG:
-
Precentral gyri
- RD:
-
Radial diffusivity
- ROI:
-
Regions of interest
- SLF:
-
Superior longitudinal fasciculus
- SMA:
-
Supplementary motor areas
- TBSS:
-
Tract-based spatial statistics
- TE:
-
Echo time
- TR:
-
Repetition time
- WM:
-
White matter
References
Agosta F, Galantucci S, Riva N, Chio A, Messina S, Iannaccone S, Calvo A, Silani V, Copetti M, Falini A, Comi G, Filippi M (2013) Intrahemispheric and interhemispheric structural network abnormalities in PLS and ALS. Hum Brain Mapp. doi:10.1002/hbm.22286
Agosta F, Pagani E, Petrolini M, Caputo D, Perini M, Prelle A, Salvi F, Filippi M (2010) Assessment of white matter tract damage in patients with amyotrophic lateral sclerosis: a diffusion tensor MR imaging tractography study. AJNR Am J Neuroradiol 31:1457–1461
Agosta F, Pagani E, Rocca MA, Caputo D, Perini M, Salvi F, Prelle A, Filippi M (2007) Voxel-based morphometry study of brain volumetry and diffusivity in amyotrophic lateral sclerosis patients with mild disability. Hum Brain Mapp 28:1430–1438
Agosta F, Valsasina P, Riva N, Copetti M, Messina MJ, Prelle A, Comi G, Filippi M (2012) The cortical signature of amyotrophic lateral sclerosis. PLoS ONE 7:e42816
Bartels C, Mertens N, Hofer S, Merboldt KD, Dietrich J, Frahm J, Ehrenreich H (2008) Callosal dysfunction in amyotrophic lateral sclerosis correlates with diffusion tensor imaging of the central motor system. Neuromuscular Disord 18:398–407
Bastin ME, Pettit LD, Bak TH, Gillingwater TH, Smith C, Abrahams S (2013) Quantitative tractography and tract shape modeling in amyotrophic lateral sclerosis. J Magn Resonance Imaging 38:1140–1145
Bede P, Bokde A, Elamin M, Byrne S, McLaughlin RL, Jordan N, Hampel H, Gallagher L, Lynch C, Fagan AJ, Pender N, Hardiman O (2013) Grey matter correlates of clinical variables in amyotrophic lateral sclerosis (ALS): a neuroimaging study of ALS motor phenotype heterogeneity and cortical focality. J Neurol Neurosurg Psychiatry 84:766–773
Behrens TE, Berg HJ, Jbabdi S, Rushworth MF, Woolrich MW (2007) Probabilistic diffusion tractography with multiple fibre orientations: what can we gain? Neuroimage 34:144–155
Brooks BR, Miller RG, Swash M, Munsat TL (2000) El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotrophic lateral sclerosis and other motor neuron disorders. Off Publ World Fed Neurol Res Group Motor Neuron Dis 1:293–299
Budde MD, Janes L, Gold E, Turtzo LC, Frank JA (2011) The contribution of gliosis to diffusion tensor anisotropy and tractography following traumatic brain injury: validation in the rat using Fourier analysis of stained tissue sections. Brain 134:2248–2260
Cedarbaum JM, Stambler N, Malta E, Fuller C, Hilt D, Thurmond B, Nakanishi A (1999) The ALSFRS-R: a revised ALS functional rating scale that incorporates assessments of respiratory function. BDNF ALS Study Group (Phase III). J Neurol Sci 169:13–21
Ciccarelli O, Behrens TE, Altmann DR, Orrell RW, Howard RS, Johansen-Berg H, Miller DH, Matthews PM, Thompson AJ (2006) Probabilistic diffusion tractography: a potential tool to assess the rate of disease progression in amyotrophic lateral sclerosis. Brain 129:1859–1871
Cirillo M, Esposito F, Tedeschi G, Caiazzo G, Sagnelli A, Piccirillo G, Conforti R, Tortora F, Monsurro MR, Cirillo S, Trojsi F (2012) Widespread microstructural white matter involvement in amyotrophic lateral sclerosis: a whole-brain DTI study. AJNR Am J Neuroradiol 33:1102–1108
Douaud G, Filippini N, Knight S, Talbot K, Turner MR (2011) Integration of structural and functional magnetic resonance imaging in amyotrophic lateral sclerosis. Brain 134:3470–3479
Ellis CM, Simmons A, Jones DK, Bland J, Dawson JM, Horsfield MA, Williams SC, Leigh PN (1999) Diffusion tensor MRI assesses corticospinal tract damage in ALS. Neurology 53:1051–1058
Filippini N, Douaud G, Mackay CE, Knight S, Talbot K, Turner MR (2010) Corpus callosum involvement is a consistent feature of amyotrophic lateral sclerosis. Neurology 75:1645–1652
Fischer LR, Culver DG, Tennant P, Davis AA, Wang M, Castellano-Sanchez A, Khan J, Polak MA, Glass JD (2004) Amyotrophic lateral sclerosis is a distal axonopathy: evidence in mice and man. Exp Neurol 185:232–240
Frey D, Schneider C, Xu L, Borg J, Spooren W, Caroni P (2000) Early and selective loss of neuromuscular synapse subtypes with low sprouting competence in motoneuron diseases. J Neurosci 20:2534–2542
Hardiman O, van den Berg LH, Kiernan MC (2011) Clinical diagnosis and management of amyotrophic lateral sclerosis. Nat Rev Neurol 7:639–649
Hua K, Zhang J, Wakana S, Jiang H, Li X, Reich DS, Calabresi PA, Pekar JJ, van Zijl PC, Mori S (2008) Tract probability maps in stereotaxic spaces: analyses of white matter anatomy and tract-specific quantification. Neuroimage 39:336–347
Iwata NK, Kwan JY, Danielian LE, Butman JA, Tovar-Moll F, Bayat E, Floeter MK (2011) White matter alterations differ in primary lateral sclerosis and amyotrophic lateral sclerosis. Brain 134:2642–2655
Julien JP (1997) Neurofilaments and motor neuron disease. Trends Cell Biol 7:243–249
Keil C, Prell T, Peschel T, Hartung V, Dengler R, Grosskreutz J (2012) Longitudinal diffusion tensor imaging in amyotrophic lateral sclerosis. BMC Neurosci 13:141
Kiernan MC, Vucic S, Cheah BC, Turner MR, Eisen A, Hardiman O, Burrell JR, Zoing MC (2011) Amyotrophic lateral sclerosis. Lancet 377:942–955
Li J, Pan P, Song W, Huang R, Chen K, Shang H (2012) A meta-analysis of diffusion tensor imaging studies in amyotrophic lateral sclerosis. Neurobiol Aging 33:1833–1838
Makris N, Kennedy DN, McInerney S, Sorensen AG, Wang R, Caviness VS Jr, Pandya DN (2005) Segmentation of subcomponents within the superior longitudinal fascicle in humans: a quantitative, in vivo, DT-MRI study. Cereb Cortex 15:854–869
Metwalli NS, Benatar M, Nair G, Usher S, Hu X, Carew JD (2010) Utility of axial and radial diffusivity from diffusion tensor MRI as markers of neurodegeneration in amyotrophic lateral sclerosis. Brain Res 1348:156–164
Meyer BU, Roricht S, Woiciechowsky C (1998) Topography of fibers in the human corpus callosum mediating interhemispheric inhibition between the motor cortices. Ann Neurol 43:360–369
Nasreddine ZS, Phillips NA, Bedirian V, Charbonneau S, Whitehead V, Collin I, Cummings JL, Chertkow H (2005) The montreal cognitive assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 53:695–699
Neary D, Snowden JS, Gustafson L, Passant U, Stuss D, Black S, Freedman M, Kertesz A, Robert PH, Albert M, Boone K, Miller BL, Cummings J, Benson DF (1998) Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology 51:1546–1554
Nichols TE, Holmes AP (2002) Nonparametric permutation tests for functional neuroimaging: a primer with examples. Hum Brain Mapp 15:1–25
Oldfield RC (1971) The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9:97–113
Passamonti L, Fera F, Tessitore A, Russo A, Cerasa A, Gioia CM, Monsurro MR, Migliaccio R, Tedeschi G, Quattrone A (2013) Dysfunctions within limbic-motor networks in amyotrophic lateral sclerosis. Neurobiol Aging 34:2499–2509
Prudlo J, Bissbort C, Glass A, Grossmann A, Hauenstein K, Benecke R, Teipel SJ (2012) White matter pathology in ALS and lower motor neuron ALS variants: a diffusion tensor imaging study using tract-based spatial statistics. J Neurol 259:1848–1859
Ringholz GM, Appel SH, Bradshaw M, Cooke NA, Mosnik DM, Schulz PE (2005) Prevalence and patterns of cognitive impairment in sporadic ALS. Neurology 65:586–590
Robberecht W, Philips T (2013) The changing scene of amyotrophic lateral sclerosis. Nat Rev Neurosci 14:248–264
Roccatagliata L, Bonzano L, Mancardi G, Canepa C, Caponnetto C (2009) Detection of motor cortex thinning and corticospinal tract involvement by quantitative MRI in amyotrophic lateral sclerosis. Amyotrophic lateral sclerosis. Off Publ World Fed Neurol Res Group Motor Neuron Dis 10:47–52
Rose S, Pannek K, Bell C, Baumann F, Hutchinson N, Coulthard A, McCombe P, Henderson R (2012) Direct evidence of intra- and interhemispheric corticomotor network degeneration in amyotrophic lateral sclerosis: an automated MRI structural connectivity study. Neuroimage 59:2661–2669
Rothstein JD (2009) Current hypotheses for the underlying biology of amyotrophic lateral sclerosis. Ann Neurol 65(Suppl 1):S3–S9
Sage CA, Van Hecke W, Peeters R, Sijbers J, Robberecht W, Parizel P, Marchal G, Leemans A, Sunaert S (2009) Quantitative diffusion tensor imaging in amyotrophic lateral sclerosis: revisited. Hum Brain Mapp 30:3657–3675
Schuster C, Kasper E, Machts J, Bittner D, Kaufmann J, Benecke R, Teipel S, Vielhaber S, Prudlo J (2013) Focal thinning of the motor cortex mirrors clinical features of amyotrophic lateral sclerosis and their phenotypes: a neuroimaging study. J Neurol 260:2856–2864
Smith SM (2002) Fast robust automated brain extraction. Hum Brain Mapp 17:143–155
Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, Watkins KE, Ciccarelli O, Cader MZ, Matthews PM, Behrens TE (2006) Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage 31:1487–1505
Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM (2004) Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 23(Suppl 1):S208–S219
Song SK, Sun SW, Ramsbottom MJ, Chang C, Russell J, Cross AH (2002) Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. Neuroimage 17:1429–1436
Sugiyama M, Takao M, Hatsuta H, Funabe S, Ito S, Obi T, Tanaka F, Kuroiwa Y, Murayama S (2013) Increased number of astrocytes and macrophages/microglial cells in the corpus callosum in amyotrophic lateral sclerosis. Neuropathology Off J Jpn Soc Neuropathol
Thivard L, Pradat PF, Lehericy S, Lacomblez L, Dormont D, Chiras J, Benali H, Meininger V (2007) Diffusion tensor imaging and voxel based morphometry study in amyotrophic lateral sclerosis: relationships with motor disability. J Neurol Neurosurg Psychiatry 78:889–892
Tsuchida A, Fellows LK (2012) Are you upset? Distinct roles for orbitofrontal and lateral prefrontal cortex in detecting and distinguishing facial expressions of emotion. Cereb Cortex 22:2904–2912
Turner MR, Grosskreutz J, Kassubek J, Abrahams S, Agosta F, Benatar M, Filippi M, Goldstein LH, van den Heuvel M, Kalra S, Lule D, Mohammadi B (2011) Towards a neuroimaging biomarker for amyotrophic lateral sclerosis. Lancet Neurol 10:400–403
van der Graaff MM, Sage CA, Caan MW, Akkerman EM, Lavini C, Majoie CB, Nederveen AJ, Zwinderman AH, Vos F, Brugman F, van den Berg LH, de Rijk MC, van Doorn PA, Van Hecke W, Peeters RR, Robberecht W, Sunaert S, de Visser M (2011) Upper and extra-motoneuron involvement in early motoneuron disease: a diffusion tensor imaging study. Brain 134:1211–1228
Verstraete E, van den Heuvel MP, Veldink JH, Blanken N, Mandl RC, Hulshoff Pol HE, van den Berg LH (2010) Motor network degeneration in amyotrophic lateral sclerosis: a structural and functional connectivity study. PLoS ONE 5:e13664
Verstraete E, Veldink JH, Hendrikse J, Schelhaas HJ, van den Heuvel MP, van den Berg LH (2012) Structural MRI reveals cortical thinning in amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry 83:383–388
Verstraete E, Veldink JH, van den Berg LH, van den Heuvel MP (2013) Structural brain network imaging shows expanding disconnection of the motor system in amyotrophic lateral sclerosis. Hum Brain Mapp. doi:10.1002/hbm.22258
Wittstock M, Wolters A, Benecke R (2007) Transcallosal inhibition in amyotrophic lateral sclerosis. Clin Neurophysiol 118:301–307
Yin X, Zhao L, Xu J, Evans AC, Fan L, Ge H, Tang Y, Khundrakpam B, Wang J, Liu S (2012) Anatomical substrates of the alerting, orienting and executive control components of attention: focus on the posterior parietal lobe. PLoS ONE 7:e50590
Acknowledgments
This study was supported by National Natural Science Foundation of China for Young Scholars (No. 81301205).
Conflicts of interest
The authors declare that they have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding authors
Additional information
J. Zhang and X. Yin contributed equally to this paper.
Rights and permissions
About this article
Cite this article
Zhang, J., Yin, X., Zhao, L. et al. Regional alterations in cortical thickness and white matter integrity in amyotrophic lateral sclerosis. J Neurol 261, 412–421 (2014). https://doi.org/10.1007/s00415-013-7215-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-013-7215-5