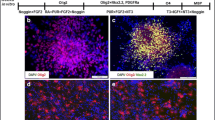
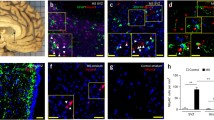
Abstract
The adult mammalian brain harbours multi-potent stem/precursor cells supporting self-renewal and differentiation within specialised niches, namely the subventricular zone of the lateral ventricles and the subgranular zone of the dentate gyrus of the hippocampus. In response to different environmental cues, these neural stem/precursor cells (NPCs) may differentiate into neurons, astrocytes or oligodendrocytes. Due to their intrinsic plasticity, these cells are considered an attractive therapeutic tool for the treatment of several neurological disorders. We have shown that syngenic NPCs, injected systemically in mice with chronic central nervous system (CNS) inflammation, reduce tissue damage and improve functional recovery. NPCs express constitutively activated integrins, which enable them to enter the CNS. Once in the site of tissue injury, transplanted NPCs promote brain repair through several mechanisms of action. They can induce apoptosis of CNS-infiltrating T cells as well as foster remyelination driven by endogenous oligodendrocyte progenitors. Neuroprotective and immunomodulatory molecules released principally from undifferentiated NPCs at the site of tissue damage mediate these effects. This bystander (or paracrine) ability of transplanted NPCs to protect the CNS from different types of injury suggests that such therapeutic procedure could be of great interest in the future therapeutic armamentarium of inflammatory demyelinating diseases of the CNS.
Similar content being viewed by others
References
Adams CW, Abdulla YH, Torres EM, Poston RN (1987) Periventricular lesions in multiple sclerosis: their perivenous origin and relationship to granular ependymitis. Neuropathol Appl Neurobiol 13:141-52
Brundin L, Brismar H, Danilov AI, Olsson T, Johansson CB (2003) Neural stem cells: a potential source for remyelination in neuroinflammatory disease. Brain Pathol 13:322-28
Danilov AI, Covacu R, Moe MC, Langmoen IA, Johansson CB, Olsson T, Brundin L (2006) Neurogenesis in the adult spinal cord in an experimental model of multiple sclerosis. Eur J Neurosci 23:394-00
De Paola V, Holtmaat A, Knott G, Song S, Wilbrecht L, Caroni P, Svoboda K (2006) Cell type-specific structural plasticity of axonal branches and boutons in the adult neocortex. Neuron 49:861-75
Defelipe J (2006) Brain plasticity and mental processes: Cajal again. Nat Rev Neurosci 7:811-17
Doetsch F (2003) The glial identity of neural stem cells. Nat Neurosci 6:1127-134
Engell T (1989) A clinical pathoanatomical study of clinically silent multiple sclerosis. Acta Neurol Scand 79:428-30
Filippi M, Campi A, Mammi S, Martinelli V, Locatelli T, Scotti G, Amadio S, Canal N, Comi G (1995) Brain magnetic resonance imaging and multimodal evoked potentials in benign and secondary progressive multiple sclerosis. J Neurol Neurosurg Psychiatry 58:31-7
Franklin RJ (2002) Why does remyelination fail in multiple sclerosis? Nat Rev Neurosci 3:705-14
Georgi W (1961) Multiple sclerosis. Anatomopathological findings of multiple sclerosis in diseases not clinically diagnosed. Schweiz Med Wochenschr 91:605-07
Gilbert JJ, Sadler M (1983) Unsuspected multiple sclerosis. Arch Neurol 40:533-36
Lee SJ, Benveniste EN (1999) Adhesion molecule expression and regulation on cells of the central nervous system. J Neuroimmunol 98:77-8
Mackay RP, Hirano A (1967) Forms of benign multiple sclerosis. Report of two “clinically silent”-cases discovered at autopsy. Arch Neurol 17:588-00
Martino G (2004) How the brain repairs itself: new therapeutic strategies in inflammatory and degenerative CNS disorders. Lancet Neurol 3:372-78
Martino G, Adorini L, Rieckmann P, Hillert J, Kallmann B, Comi G, Filippi M (2002) Inflammation in multiple sclerosis: the good, the bad, and the complex. Lancet Neurol 1:499-09
Martino G, Pluchino S (2006) The therapeutic potential of neural stem cells. Nat Rev Neurosci 7:395-06
Menn B, Garcia-Verdugo JM, Yaschine C, Gonzalez-Perez O, Rowitch D, Alvarez-Buylla A (2006) Origin of oligodendrocytes in the subventricular zone of the adult brain. J Neurosci 26:7907-918
Monje ML, Toda H, Palmer TD (2003) Inflammatory blockade restores adult hippocampal neurogenesis. Science 302:1760-765
Patrikios P, Stadelmann C, Kutzelnigg A, Rauschka H, Schmidbauer M, Laursen H, Sorensen PS, Bruck W, Lucchinetti C, Lassmann H (2006) Remyelination is extensive in a subset of multiple sclerosis patients. Brain 129:3165-172
Picard-Riera N, Decker L, Delarasse C, Goude K, Nait-Oumesmar B, Liblau R, Pham-Dinh D, Evercooren AB (2002) Experimental autoimmune encephalomyelitis mobilizes neural progenitors from the subventricular zone to undergo oligodendrogenesis in adult mice. Proc Natl Acad Sci USA 99:13211-3216
Pluchino S, Quattrini A, Brambilla E et al. (2003) Injection of adult neurospheres induces recovery in a chronic model of multiple sclerosis. Nature 422:688-94
Pluchino S, Zanotti L, Rossi B et al. (2005) Neurosphere-derived multipotent precursors promote neuroprotection by an immunomodulatory mechanism. Nature 436:266-71
Sanai N, Tramontin AD, Quinones-Hinojosa A et al. (2004) Unique astrocyte ribbon in adult human brain contains neural stem cells but lacks chain migration. Nature 427:740-44
Weinshenker BG (1995) The natural history of multiple sclerosis. Neurol Clin 13:119-46
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pluchino, S., Zanotti, L. & Martino, G. Rationale for the use of neural stem/precursor cells in immunemediated demyelinating disorders. J Neurol 254 (Suppl 1), I23–I28 (2007). https://doi.org/10.1007/s00415-007-1005-x
Issue Date:
DOI: https://doi.org/10.1007/s00415-007-1005-x