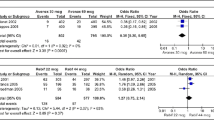
Abstract
Neutralising antibodies develop in up to 40% of patients treated with interferon (IFN)-β. The frequency of detection of such antibodies depends on the sensitivity of the assay, as well as on the preparation, dose, frequency and route of administration of the IFN-β. The lowest frequency of antibody development is seen with IFN-β 1a im, whereas antibodies appear to develop faster with IFN-β 1b sc than with IFN-β 1a sc. After two to three years of continuous therapy, the proportion of neutralising antibody positive patients tends to decrease, although patients who have been seropositive for over twelve months usually remain seropositive for several years to come. The presence of neutralising antibodies diminishes, and at high titres, even abolishes the bioavailability and in vivo biological response to IFN-β. For this reason, neutralising antibodies reduce the clinical impact of treatment on relapse rate, time to first relapse, disease activity measured by magnetic resonance imaging and, in the long-term, accumulation of disability. Recent guidelines recommend regular antibody testing in all patients treated with IFN-β, discontinuation of IFN-β treatment in patients with sustained high neutralising antibodies titres, and switch to an alternative therapy if appropriate.
Similar content being viewed by others
References
Clanet M, Radue EW, Kappos L et al. (2002) A randomized, double-blind, dose-comparison study of weekly interferon beta-1a in relapsing MS. Neurology 59:1507–1517
Durelli L, Verdun E, Barbero P et al. (2002) Every-other-day interferon beta-1b versus once-weekly interferon beta-1a for multiple sclerosis: results of a 2-year prospective randomised multicentre study (INCOMIN). Lancet 359:1453–1460
European Study Group on interferon beta-1b in secondary progressive MS (1998) Placebo-controlled multicentre randomised trial of interferon beta-1b in treatment of secondary progressive multiple sclerosis. Lancet 352:1491–1497
Jacobs LD, Cookfair DL, Rudick RA et al. (1996) Intramuscular interferon beta-1a for disease progression in relapsing multiple sclerosis. The Multiple Sclerosis Collaborative Research Group (MSCRG). Ann Neurol 39:285–294
Kappos L, Clanet M, Sandberg-Wolheim M et al. (2005) Neutralizing antibodies and efficacy of interferon beta-1a: a 4-year controlled study. Neurology 65:40–47
Panitch H, Goodin DS, Francis G et al. (2002) Randomized, comparative study of interferon beta-1a treatment regimens in MS: The EVIDENCE Trial. Neurology 59:1496–1506
Petersen B, Bendtzen K, Koch-Henriksen N, Ravnborg M, Ross C, Sorensen PS; Danish Multiple Sclerosis Group (2006) Persistence of neutralizing antibodies after discontinuation of IFN-beta therapy in patients with relapsing-remitting multiple sclerosis. Mult Scler 12(3):247–252
Polman C, Kappos L, White R et al. (2003) Neutralizing antibodies during treatment of secondary progressive MS with interferon beta-1b. Neurology 60:37–43
PRISMS Study Group (2001) PRISMS-4: Long-term efficacy of interferon-beta-1a in relapsing MS. Neurology 56:1628–1636
Rice GP, Paszner B, Oger J, Lesaux J, Paty D, Ebers G (1999) The evolution of neutralizing antibodies in multiple sclerosis patients treated with interferon beta-1b. Neurology 52:1277–1279
Ross C, Clemmesen KM, Svenson M, Sørensen PS, Koch-Henriksen N, Skovgaard GL et al. (2000) Immunogenicity of interferon-beta in multiple sclerosis patients: influence of preparation, dosage, dose frequency, and route of administration. Danish Multiple Sclerosis Study Group. Ann Neurol 48:706–712
Rudick RA, Simonian NA, Alam JA et al. (1998) Incidence and significance of neutralizing antibodies to interferon beta-1a in multiple sclerosis.Multiple Sclerosis Collaborative Research Group (MSCRG). Neurology 50:1266–1272
Sørensen PS, Ross C, Clemmesen KM et al. (2003) Clinical importance of neutralising antibodies against interferon beta in patients with relapsing–remitting multiple sclerosis. Lancet 362:1184–1191
Sørensen PS, Koch-Henriksen N, Ross C, Clemmesen KM, Bendtzen K (2005) Appearance and disappearance of neutralizing antibodies during interferonbeta therapy. Neurology 65:33–39
Sørensen PS, Deisenhammer F, Duda P, Hohlfeld R, Myhr KM, Palace J, Polman C, Pozzilli C, Ross C; EFNS Task Force on Anti-IFN-beta Antibodies in Multiple Sclerosis (2005) Guidelines on use of anti-IFN-beta antibody measurements in multiple sclerosis: report of an EFNS Task Force on IFN-beta antibodies in multiple sclerosis. Eur J Neurol 12(11):817–827
SPECTRIMS (Secondary Progressive Efficacy Clinical Trial of Recombinant Interferon-beta-1a in MS) Study Group (2001) Randomized controlled trial of interferon-beta-1a in secondary progressive MS: clinical results. Neurology 56:1496–1504
The IFNB Multiple Sclerosis Study Group (1993) Interferon beta-1b is effective in relapsing-remitting multiple sclerosis. I. Clinical results of a multi-center, randomized, double-blind, placebo-controlled trial. Neurology 43:655–661
The IFNB Multiple Sclerosis Study Group and The University of British Columbia MS/MRI Analysis Group (1996) Neutralizing antibodies during treatment of multiple sclerosis with interferon beta-1b: experience during the first three years. Neurology 47:889–894
The North American Study Group on interferon beta-1b in Secondary Progressive MS (2004) Interferon beta-1b in secondary progressive MS: Results from a 3-year controlled study. Neurology 63:1788–1795
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sørensen, P.S. Neutralising antibodies to interferon-β–measurement, clinical relevance, and management. J Neurol 253 (Suppl 6), vi16–vi22 (2006). https://doi.org/10.1007/s00415-006-6004-9
Issue Date:
DOI: https://doi.org/10.1007/s00415-006-6004-9