Abstract
Objective
To test the hypothesis that patients with amyotrophic lateral sclerosis (ALS) show increased cortical activation during a motor task compared to both healthy controls and patients with muscle weakness due to peripheral lesions.
Methods
Functional magnetic resonance imaging (fMRI) was used to measure activation during a block design paradigm contrasting right hand movements against rest in sixteen patients with ALS, seventeen healthy controls and nine patients with peripheral lesions. The groups were matched for age and gender and the two patient groups were matched for their degree of upper limb weakness. Analysis used a non-parametric approach to perform a 3 way hypothesis-driven comparison between the groups.
Results
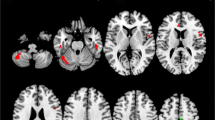
During the motor task, patients with ALS showed increased cortical activation bilaterally, extending from the sensorimotor cortex [Brodmann areas (BA) 1, 2, 4] posteriorly into the inferior parietal lobule (BA 40) and inferiorly to the superior temporal gyrus (BA 22) when compared to peripheral lesion patients and controls. In addition, ALS patients showed reduced activation in the dorsolateral prefrontal cortex (DLPFC) extending to anterior and medial frontal cortex (BA 8, 9, 10, 32).
Conclusions
We conclude that alterations in cortical function in ALS differ in sensorimotor and prefrontal regions. Importantly, we have shown that these changes do not reflect confounding by weakness or task difficulty, but are likely to be related to upper motor neuron pathology in ALS.
Similar content being viewed by others
References
Brownell B, Oppenheimer DR, Hughes JT (1970) The central nervous system in motor neurone disease. J Neurol Neurosurg Psychiatry 33:338–357
Ince PG, Evans J, Knopp M, et al. (2003) Corticospinal tract degeneration in the progressive muscular atrophy variant of ALS. Neurology 60:1252–1258
Piao YS, Wakabayashi K, Kakita A, et al. (2003) Neuropathology with clinical correlations of sporadic amyotrophic lateral sclerosis: 102 autopsy cases examined between 1962 and 2000. Brain Pathol 13:10–22
Kew JJ, Leigh PN, Playford ED, et al. (1993) Cortical function in amyotrophic lateral sclerosis. A positron emission tomography study. Brain 116:655–680
Kew JJ, Brooks DJ, Passingham RE, Rothwell JC, Frackowiak RS, Leigh PN (1994) Cortical function in progressive lower motor neuron disorders and amyotrophic lateral sclerosis: a comparative PET study. Neurology 44:1101–1110
Konrad C, Henningsen H, Bremer J, et al. (2002) Pattern of cortical reorganization in amyotrophic lateral sclerosis: a functional magnetic resonance imaging study. Exp Brain Res 143:51–56
Schoenfeld MA, Tempelmann C, Gaul C, et al. (2005) Functional motor compensation in amyotrophic lateral sclerosis. J Neurol 252:944–952
Konrad C, Jansen A, Henningsen H, et al. (2006) Subcortical reorganization in amyotrophic lateral sclerosis. Exp Brain Res 172(3):361–369
Leigh PN, Abrahams S, Al Chalabi A, et al. (2003) The management of motor neurone disease. J Neurol Neurosurg Psychiatry 74(Suppl 4):iv32–iv47
Brooks BR, Miller RG, Swash M, Munsat TL (2000) El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord 1:293–299
Kleyweg RP, van der Meche FG, Schmitz PI (1991) Interobserver agreement in the assessment of muscle strength and functional abilities in Guillain-Barre syndrome. Muscle Nerve 14:1103–1109
Cedarbaum JM, Stambler N, Malta E, et al. (1999) The ALSFRS-R: a revised ALS functional rating scale that incorporates assessments of respiratory function. BDNF ALS Study Group (Phase III). J Neurol Sci 169:13–21
Evans FJ (1978) Monitoring attention deployment by random number generation: an index to measure subjective randomness. Bull Psychonom Soc 12:35–38
Towse JN, Neil D (1998) Analyzing human random generation behaviour: a review of methods used and a computer programme for describing performance. Behav Res Methods Instrum Comput 30:583–591
Brammer MJ, Bullmore ET, Simmons A, et al. (1997) Generic brain activation mapping in functional magnetic resonance imaging: a nonparametric approach. Magn Reson Imaging 15:763–770
Bullmore E, Brammer M, Williams SC, et al. (1996) Statistical methods of estimation and inference for functional MR image analysis. Magn Reson Med 35:261–277
Bullmore E, Long C, Suckling J, et al. (2001) Colored noise and computational inference in neurophysiological (fMRI) time series analysis: resampling methods in time and wavelet domains. Hum Brain Mapp 12:61–68
Bullmore ET, Brammer MJ, Rabe-Hesketh S, et al. (1999) Methods for diagnosis and treatment of stimulus-correlated motion in generic brain activation studies using fMRI. Hum Brain Mapp 7:38–38
Bullmore ET, Suckling J, Overmeyer S, Rabe-Hesketh S, Taylor E, Brammer MJ (1999) Global, voxel, and cluster tests, by theory and permutation, for a difference between two groups of structural MR images of the brain. IEEE Trans Med Imaging 18:32–42
Talairach J, Tornoux P (1988) CoPlanar Sterotaxic Atlas of the human brain. Thieme, Stuttgart
Hirano A (1991) Cytopathology of amyotrophic lateral sclerosis. Adv Neurol 56:91–101
Hughes JT (1982) Pathology of amyotrophic lateral sclerosis. Adv Neurol 36:61–74
Friedman AP, Freedman D (1950) Amyotrophic lateral sclerosis. J Nerv Ment Dis 111:1–18
Kiernan JA, Hudson AJ (1991) Changes in sizes of cortical and lower motor neurons in amyotrophic lateral sclerosis. Brain 114:843–853
Martin JE, Swash M (1995) The pathology of motor neuron disease. In: Swash M, Leigh PN (eds) Motor neuron disease: biology and management. Springer-Verlag, London, p 93
Maekawa S, Al Sarraj S, Kibble M, et al. (2004) Cortical selective vulnerability in motor neuron disease: a morphometric study. Brain 127:1237–1251
Chollet F, DiPiero V, Wise RJ, Brooks DJ, Dolan RJ, Frackowiak RS (1991) The functional anatomy of motor recovery after stroke in humans: a study with positron emission tomography. Ann Neurol 29:63–71
Muller RA, Rothermel RD, Behen ME, Muzik O, Mangner TJ, Chugani HT (1998) Differential patterns of language and motor reorganization following early left hemisphere lesion: a PET study. Arch Neurol 55:1113–1119
Weiller C, Chollet F, Friston KJ, Wise RJ, Frackowiak RS (1992) Functional reorganization of the brain in recovery from striatocapsular infarction in man. Ann Neurol 31:463–472
Wunderlich G, Knorr U, Herzog H, Kiwit JC, Freund HJ, Seitz RJ (1998) Precentral glioma location determines the displacement of cortical hand representation. Neurosurgery 42:18–26
Neary D, Snowden J (1996) Frontotemporal dementia: nosology, neuropsychology, and neuropathology. Brain Cogn 31:176–187
Shaw PJ (1994) Excitotoxicity and motor neurone disease: a review of the evidence. J Neurol Sci 124(Suppl):6–13
Neary D, Snowden JS, Mann DM (2000) Cognitive change in motor neurone disease/amyotrophic lateral sclerosis (MND/ALS). J Neurol Sci 180:15–20
Abrahams S, Leigh PN, Harvey A, Vythelingum GN, Grise D, Goldstein LH (2000) Verbal fluency and executive dysfunction in amyotrophic lateral sclerosis (ALS). Neuropsychologia 38:734–747
Gallassi R, Montagna P, Ciardulli C, Lorusso S, Mussuto V, Stracciari A (1985) Cognitive impairment in motor neuron disease. Acta Neurol Scand 71:480–484
Kew JJ, Goldstein LH, Leigh PN, et al. (1993) The relationship between abnormalities of cognitive function and cerebral activation in amyotrophic lateral sclerosis. A neuropsychological and positron emission tomography study. Brain 116:1399–1423
Ludolph AC, Langen KJ, Regard M, et al. (1992) Frontal lobe function in amyotrophic lateral sclerosis: a neuropsychologic and positron emission tomography study. Acta Neurol Scand 85:81–89
Abrahams S, Goldstein LH, Simmons A, et al. (2004) Word retrieval in amyotrophic lateral sclerosis: a functional magnetic resonance imaging study. Brain 127:1507–1517
Jahanshahi M, Jenkins IH, Brown RG, Marsden CD, Passingham RE, Brooks DJ (1995) Self-initiated versus externally triggered movements. I. An investigation using measurement of regional cerebral blood flow with PET and movement-related potentials in normal and Parkinson’s disease subjects. Brain 118:913–933
Frith CD, Friston K, Liddle PF, Frackowiak RS (1991) Willed action and the prefrontal cortex in man: a study with PET. Proc Biol Sci 244:241–246
Frith CD, Friston KJ, Liddle PF, Frackowiak RS (1991) A PET study of word finding. Neuropsychologia 29:1137–1148
Ellis CM, Suckling J, Amaro E Jr, et al. (2001) Volumetric analysis reveals corticospinal tract degeneration and extramotor involvement in ALS. Neurology 57:1571–1578
Abrahams S, Goldstein LH, Suckling J, et al. (2005) Frontotemporal white matter changes in amyotrophic lateral sclerosis. J Neurol 252:321–331
Abe K, Takanashi M, Watanabe Y, et al. (2001) Decrease in N-acetylaspartate/creatine ratio in the motor area and the frontal lobe in amyotrophic lateral sclerosis. Neuroradiology 43:537–541
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Stanton, B.R., Williams, V.C., Leigh, P.N. et al. Altered cortical activation during a motor task in ALS. J Neurol 254, 1260–1267 (2007). https://doi.org/10.1007/s00415-006-0513-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-006-0513-4