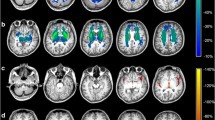
Abstract
Wilson’s disease (WD) is characterized by impaired hepatic copper secretion and subsequent copper accumulation in many organs predominantly liver and brain, secondary to loss of function mutations in the copper transport protein ATP7B. If the disease is recognized too late or treatment is not adequate, brain copper accumulation leads to progressive neurodegeneration with a variety of clinical symptoms. The nigrostriatal dopaminergic system seems rather vulnerable. Midbrain atrophy, however, has not been recognized as one of the prime features of patients with WD.
Here we report quantification of midbrain diameter in 41 patients with WD. Data were correlated to the severity of neurological symptoms and the integrity of dopaminergic neurons measured via dopamine transporter binding. For control, we measured midbrain diameter in 18 patients with no evidence for brainstem dysfunction and 5 patients with progressive supranuclear palsy (PSP).
Patients with WD had a reduced midbrain diameter (15.5 ± 0.4 mm) compared to controls (18.5 ± 0.2 mm). WD patients without neurological symptoms had midbrain diameter that were not different from controls (18.0 ± 0.3 mm), while patients with neurological symptoms showed midbrain atrophy similar to patients with PSP (14.4 ± 0.3 mm versus 14.1 ± 0.3). There was a strong and significant correlation between midbrain atrophy and the severity of neurological symptoms (r= −0.68, p < 0.001) while midbrain atrophy and dopamine transporter binding correlated significantly but was less pronounced (r=0.46, p < 0.001).
In summary, we were able to show, that midbrain diameter is an easy to perform quantification of neurodegeneration induced by brain copper accumulation and that other structures than substantia nigra dopaminergic neurons seem to contribute to midbrain atrophy in WD.




Similar content being viewed by others
References
Adachi M, Kawanami T, Ohshima H, Sugai Y, Hosoya T (2004) Morning glory sign: a particular MR finding in progressive supranuclear palsy. Magn Reson Med Sci 3:125–132
Arnold G, Tatsch K, Kraft E, Oertel WH, Schwarz J (2002) Steele-Richardson-Olszewski-syndrome: reduction of dopamine D2 receptor binding relates to the severity of midbrain atrophy in vivo: (123)IBZM SPECT and MRI study. Mov Disord 17:557–562
Barthel H, Hermann W, Kluge R, Hesse S, Collingridge DR, Wagner A, Sabri O (2003) Concordant pre- and postsynaptic deficits of dopaminergic neurotransmission in neurologic Wilson disease. AJNR Am J Neuroradiol 24:234–238
Barthel H, Sorger D, Kuhn HJ, Wagner A, Kluge R, Hermann W (2001) Differential alteration of the nigrostriatal dopaminergic system in Wilson’s disease investigated with [123I]ss-CIT and high-resolution SPET. Eur J Nucl Med 28:1656–1663
Brewer GJ, Yuzbasiyan-Gurkan V (1992) Wilson disease. Medicine 71:139–164
Caca K, Ferenci P, Kuhn HJ, Polli C, Willgerodt H, Kunath B, Hermann W, Mossner J, Berr F (2001) High prevalence of the H1069Q mutation in East German patients with Wilson disease: rapid detection of mutations by limited sequencing and phenotype-genotype analysis. J Hepatol 35:575–581
Engelbrecht V, Schlaug G, Hefter H, Kahn T, Modder U (1995) MRI of the brain in Wilson disease: T2 signal loss under therapy. J Comput Assist Tomogr 19:635–638
Hermann W, Barthel H, Hesse S, Grahmann F, Kuhn HJ, Wagner A, Villmann T (2002) Comparison of clinical types of Wilson’s disease and glucose metabolism in extrapyramidal motor brain regions. J Neurol 249:896–901
Hermann W, Gunther P, Hahn S, Dietrich J, Villmann T, Eggers B, Wagner A (2002) [Cerebral MRI and evoked potentials in Wilson disease. Comparison of findings in patients with neurological follow-up]. Nervenarzt 73:349–354
Hermann W, Villmann T, Wagner A (2003) [Electrophysiological impairment profile of patients with Wilson’s disease]. Nervenarzt 74:881–887
Hitoshi S, Iwata M, Yoshikawa K (1991) Mid-brain pathology of Wilson’s disease: MRI analysis of three cases. J Neurol Neurosurg Psychiatry 54:624–626
Huang CC, Chu NS, Yen TC, Wai YY, Lu CS (2003) Dopamine transporter binding in Wilson’s disease. Can J Neurol Sci 30:163–167
Jeon B, Kim JM, Jeong JM, Kim KM, Chang YS, Lee DS, Lee MC (1998) Dopamine transporter imaging with [123I]-beta-CIT demonstrates presynaptic nigrostriatal dopaminergic damage in Wilson’s disease. J Neurol Neurosurg Psychiatry 65:60–64
Kato N, Arai K, Hattori T (2003) Study of the rostral midbrain atrophy in progressive supranuclear palsy. J Neurol Sci 210:57–60
Kraft E, Trenkwalder C, Then Bergh F, Auer DP (1999) Magnetic resonance proton spectroscopy of the brain in Wilson’s disease. J Neurol 246:693–699
Leggio L, Addolorato G, Abenavoli L, Gasbarrini G (2005) Wilson’s disease: clinical, genetic and pharmacological findings. Int J Immunopathol Pharmacol 18:7–14
Metz CE, Pan X (1999) “Proper” Binormal ROC Curves: Theory and Maximum-Likelihood Estimation. J Math Psychol 43:1–33
Oertel WH, Tatsch K, Schwarz J, Kraft E, Trenkwalder C, Scherer J, Weinzierl M, Vogl T, Kirsch CM (1992) Decrease of D2 receptors indicated by 123I-iodobenzamide single-photon emission computed tomography relates to neurological deficit in treated Wilson’s disease. Ann Neurol 32:743–748
Page RA, Davie CA, MacManus D, Miszkiel KA, Walshe JM, Miller DH, Lees AJ, Schapira AH (2004) Clinical correlation of brain MRI and MRS abnormalities in patients with Wilson disease. Neurology 63:638–643
Righini A, Antonini A, De Notaris R, Bianchini E, Meucci N, Sacilotto G, Canesi M, De Gaspari D, Triulzi F, Pezzoli G (2004) MR imaging of the superior profile of the midbrain: differential diagnosis between progressive supranuclear palsy and Parkinson disease AJNR. Am J Neuroradiol 25:927–932
Schwarz J, Antonini A, Kraft E, Tatsch K, Vogl T, Kirsch CM, Leenders KL, Oertel WH (1994) Treatment with D-penicillamine improves dopamine D2-receptor binding and T2-signal intensity in de novo Wilson’s disease. Neurology 44:1079–1082
Schwarz J, Storch A, Koch W, Pogarell O, Radau PE, Tatsch K (2004) Loss of dopamine transporter binding in Parkinson’s disease follows a single exponential rather than linear decline. J Nucl Med 45:1694–1697
Schwarz J, Tatsch K, Vogl T, Kirsch CM, Trenkwalder C, Arnold G, Gasser T, Oertel WH (1992) Marked reduction of striatal dopamine D2 receptors as detected by 123IBZM-SPECT in a Wilson’s disease patient with generalized dystonia. Mov Disord 7:58–61
Shah AB, Chernov I, Zhang HT, Ross BM, Das K, Lutsenko S, Parano E, Pavone L, Evgrafov O, Ivanova-Smolenskaya IA, Anneren G, Westermark K, Urrutia FH, Penchaszadeh GK, Sternlieb I, Scheinberg IH, Gilliam TC, Petrukhin K (1997) Identification and analysis of mutations in the Wilson disease gene (ATP7B): population frequencies, genotype-phenotype correlation, and functional analyses. Am J Hum Genet 61:317–328
Snow BJ, Bhatt M, Martin WR, Li D, Calne DB (1991) The nigrostriatal dopaminergic pathway in Wilson’s disease studied with positron emission tomography. J Neurol Neurosurg Psychiatry 54:12–17
Starosta-Rubinstein S, Young AB, Kluin K, Hill G, Aisen AM, Gabrielsen T, Brewer GJ (1987) Clinical assessment of 31 patients with Wilson’s disease. Correlations with structural changes on magnetic resonance imaging. Arch Neurol 44:365–370
Tanzi RE, Petrukhin K, Chernov I, Pellequer JL, Wasco W, Ross B, Romano DM, Parano E, Pavone L, Brzustowicz LM, et al. (1993) The Wilson disease gene is a copper transporting ATPase with homology to the Menkes disease gene. Nat Genet 5:344–350
Tao TY, Gitlin JD (2003) Hepatic copper metabolism: insights from genetic disease. Hepatology 37:1241–1247
Tatsch K, Schwarz J, Mozley PD, Linke R, Pogarell O, Oertel WH, Fieber RS, Hahn K, Kung HF (1997) Relationship between clinical features of Parkinson’s disease and presynaptic dopamine transporter binding assessed with [123I]IPT and single-photon emission tomography. Eur J Nucl Med 24:415–421
Tatsch K, Schwarz J, Oertel WH, Kirsch CM (1991) SPECT imaging of dopamine D2 receptors with 123I-IBZM: initial experience in controls and patients with Parkinson’s syndrome and Wilson’s disease. Nucl Med Commun 12:699–707
van Wassenaer-van Hall HN, van den Heuvel AG, Jansen GH, Hoogenraad TU, Mali WP (1995) Cranial MR in Wilson disease: abnormal white matter in extrapyramidal and pyramidal tracts AJNR Am J Neuroradiol 16:2021–2027
Verma A, Singh NN, Misra S (2004) Early white matter changes in Wilson disease. J Assoc Physicians India 52:578–579
Warmuth-Metz M, Naumann M, Csoti I, Solymosi L (2001) Measurement of the midbrain diameter on routine magnetic resonance imaging: a simple and accurate method of differentiating between Parkinson disease and progressive supranuclear palsy. Arch Neurol 58:1076–1079
Watanabe N, Seto H, Shimizu M, Kageyama M, Morijiri M, Nomura K, Wu YW, Kakishita M (1995) Brain SPECT in Wilson’s disease. Clin Nucl Med 20:1029–1030
Wilson SAK (1912) Progressive lenticular degeneration: a familiar nervous disease associated with cirrhosis of the liver. Lancet 1:115
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Strecker, K., Schneider, J., Barthel, H. et al. Profound midbrain atrophy in patients with Wilson’s disease and neurological symptoms?. J Neurol 253, 1024–1029 (2006). https://doi.org/10.1007/s00415-006-0151-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-006-0151-x