Abstract
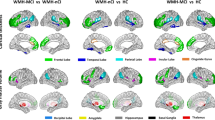
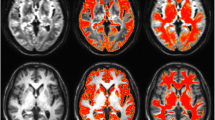
White matter hyperintensities (WMHs) are a common finding in normal elderly persons. We studied the biological damage associated with WMHs by assessing the correspondence between WMH location and regional gray matter loss.Voxel–based morphometry of the gray matter was carried out with statistical parametric mapping on high resolution MR images.Neurologically intact persons with mainly anterior (frontal>parieto–occipital; N = 39) and mainly posterior WMHs (parieto– occipital>frontal; N = 14) were compared with a group devoid of WMHs (N = 80). Subjects with mainly frontal WMHs had bilateral frontal (medial, superior, and inferior gyri) atrophy in gray matter, while subjects with mainly posterior WMHs had more diffuse atrophy, involving mainly the frontal but also the right insular region. Our findings suggest that frontal WMHs are associated with frontal gray matter damage while parietooccipital WMHs seem to have a weaker and more diffuse impact on gray matter.
Similar content being viewed by others
References
Amodio P,Wenin H, Del Piccolo F, Mapelli D,Montagnese S, Pellegrini A, Musto C, Gatta A,Umilta C (2002) Variability of trail making test, symbol digit test and line trait test in normal people. A normative study taking into account age–dependent decline and sociobiological variables. Aging Clin Exp Res 14:117–131
Ashburner J, Friston KJ (2001) Why voxel–based morphometry should be used. NeuroImage 14:1238–1243
Augustine JR (1996) Circuitry and functional aspects of the insular lobe in primates including humans. Brain Res Brain Res Rev 22:229–244
Augustine JR (1985) The insular lobe in primates including humans. Neurol Res 7:2–10
Boone KB,Miller BL, Lesser IM, Mehringer CM,Hill–Gutierrez E, Goldberg MA, Berman NG (1992) Neuropsychological correlates of white–matter lesions in healthy elderly subjects. A threshold effect. Arch Neurol 49:549–554
Caffarra P,Vezzadini G,Dieci F, Zonato F, Venneri A (2002) Rey–Osterrieth complex figure: normative values in an Italian population sample. Neurol Sci 22:443–437
Capizzano AA,Acion L, Bekinschtein T, Furman M,Gomila H,Martinez A, Mizrahi R, Starkstein SE (2004) White matter hyperintensities are significantly associated with cortical atrophy in Alzheimer’s disease. J Neurol Neurosurg Psychiatry 75:822–827
Carlesimo GA, Caltagirone C, Gainotti G (1996) The Mental Deterioration Battery: normative data, diagnostic reliability and qualitative analyses of cognitive impairment. The Group for the Standardization of the Mental Deterioration Battery. Eur Neurol 36:378–384
Coffey CE,Wilkinson WE, Parashos IA, Soady SA, Sullivan RJ, Patterson LJ, Figiel GS,Webb MC, Spritzer CE, Djang WT (1992) Quantitative cerebral anatomy of the aging human brain: a cross–sectional study using magnetic resonance imaging. Neurology 42:527–536
De Leeuw F, De Groot J, Breteler M White matter changes. Frequency and risk factors. In: Pantoni L (ed) The matter of white matter.Academic Pharmaceutical Production,Utrecht, p 19
DeCarli C,Murphy DG, Tranh M, Grady CL,Haxby JV,Gillette JA, Salerno JA,Gonzales–Aviles A,Horwitz B, Rapoport SI, et al. (1995) The effect of white matter hyperintensity volume on brain structure, cognitive performance, and cerebral metabolism of glucose in 51 healthy adults. Neurology 45:2077–2084
De Renzi E,Vignolo LA (1962) The token test: a sensitive test to detect receptive disturbances in aphasia. Brain 85:665–678
Evans AC,Kamber M, Collins DL, Macdonald D (1994) An MRI–based probabilistic atlas of neuroanatomy. In: Shorvon S, Fish D, Andermann F, et al. (eds) Magnetic resonance scanning and epilepsy.New York: Plenum Press, pp 263–274
Fazekas F, Schmidt R,Kleinert R, Kapeller P, Roob G, Flooh E (1998) The spectrum of age–associated brain abnormalities: their measurement and histopathological correlates. J Neural Transm Suppl 53:31–39
Folstein MF, Folstein SE,McHugh PR (1975) “Mini–mental State”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 12:189–198
Good CD, Johnsrude IS,Ashburner J, Henson RN, Friston KJ, Frackowiak RS (2001) A voxel–based morphometric study of ageing in 465 normal adult human brains. NeuroImage 14:21–36
Gootjes L, Teipel SJ, Zebuhr Y, Schwarz R, Leinsinger G, Scheltens P,Moller HJ, Hampel H (2004) Regional Distribution ofWhite Matter Hyperintensities in Vascular Dementia, Alzheimer’s Disease and Healthy Aging. Dement Geriatr Cogn Disord 18:180–188
Gunning–Dixon FM, Raz N (2000) The cognitive correlates of white matter abnormalities in normal aging: a quantitative review. Neuropsychology 14:224–232
Hunt AL, Orrison WW,Yeo RA, Haaland KY, Rhyne RL, Garry PJ, Rosenberg GA (1989) Clinical significance of MRI white matter lesions in the elderly. Neurology 39:1470–1474
Jernigan TL, Archibald SL, Fennema– Notestine C, Gamst AC, Stout JC, Bonner J,Hesselink JR (2001) Effects of age on tissues and regions of the cerebrum and cerebellum. Neurobiol Aging 22:581–594
Matsubayashi K, Shimada K, Kawamoto A, Ozawa T (1992) Incidental brain lesions on magnetic resonance imaging and neurobehavioral functions in the apparently healthy elderly. Stroke 23:175–180
Metitieri T, Geroldi C, Pezzini A, Frisoni GB, Bianchetti A, Trabucchi M (2001) The Itel–MMSE: an Italian telephone version of the Mini–Mental State Examination. Int J Geriatr Psychiatry 16:166–167
Mirsen TR, Lee DH,Wong CJ,Diaz JF, Fox AJ,Hachinski VC,Merskey H (1991) Clinical correlates of whitematter changes on magnetic resonance imaging scans of the brain. Arch Neurol 48:1015–1021
Roman GC (1987) Senile dementia of the Binswanger type. A vascular form of dementia in the elderly. JAMA 258:1782–1788
Schmidt R, Fazekas F, Offenbacher H, Dusek T, Zach E, Reinhart B,Grieshofer P, Freidl W, Eber B, Schumacher M, et al. (1993) Neuropsychologic correlates of MRI white matter hyperintensities: a study of 150 normal volunteers. Neurology 43:2490–2494
Spinnler H, Tognoni G (1987). Standardizzazione e taratura italiana di test neuropsicologici. Ital J Neurol Sci 6(Suppl 8):1–120
Sultzer DL,Mahler ME, Cummings JL, Van Gorp WG,Hinkin CH, Brown C (1995) Cortical abnormalities associated with subcortical lesions in vascular dementia. Clinical and position emission tomographic findings. Arch Neurol 52:773–780
Takahashi W, Takagi S, Ide M, Shohtsu A, Shinohara Y (2000) Reduced cerebral glucose metabolism in subjects with incidental hyperintensities on magnetic resonance imaging. J Neurol Sci 176:21–27
Tullberg M, Fletcher E, DeCarli C, Mungas D, Reed BR,Harvey DJ,Weiner MW, Chui HC, Jagust WJ (2004) White matter lesions impair frontal lobe function regardless of their location. Neurology 63:246–253
Tupler LA, Coffey CE, Logue PE, Djang WT, Fagan SM (1922) Neuropsychological importance of subcortical white matter hyperintensity. Arch Neurol 49:1248–1252
Victor M, Ropper AH (eds) (2002) Adams and Victor’s Principles Of Neurology, 7th edition.New York:McGraw– Hill
Wahlund LO, Barkhof F, Fazekas F, Bronge L,Augustin M, Sjogren M, Wallin A,Ader H, Leys D, Pantoni L, Pasquier F, Erkinjuntti T, Scheltens P; European Task Force on Age–Related White Matter Changes (2001) European Task Force on Age–Related White Matter Changes. A new rating scale for age–related white matter changes applicable to MRI and CT. Stroke 32:1318–1322
Wen W, Sachdev P, Shnier R, Brodaty H (2004) Effect of white matter hyperintensities on cortical cerebral blood volume using perfusion MRI. Neuroimage 21:1350–1356
Ylikoski A, Erkinjuntti T, Raininko R, Sarna S, Sulkava R, Tilvis R (1995) White matter hyperintensities on MRI in the neurologically nondiseased elderly. Analysis of cohorts of consecutive subjects aged 55 to 85 years living at home. Stroke 26:1171–1177
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rossi, R., Boccardi, M., Sabattoli, F. et al. Topographic correspondence between white matter hyperintensities and brain atrophy. J Neurol 253, 919–927 (2006). https://doi.org/10.1007/s00415-006-0133-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-006-0133-z