Abstract
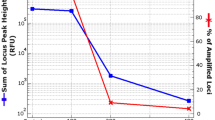
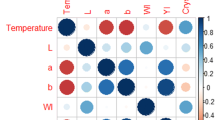
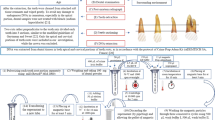
Teeth are frequently used for human identification from burnt remains, as the structure of a tooth is resilient against heat exposure. The intricate composition of hydroxyapatite (HA) mineral and collagen in teeth favours DNA preservation compared to soft tissues. Regardless of the durability, the integrity of the DNA structure in teeth can still be disrupted when exposed to heat. Poor DNA quality can negatively affect the success of DNA analysis towards human identification. The process of isolating DNA from biological samples is arduous and costly. Thus, an informative pre-screening method that could aid in selecting samples that can potentially yield amplifiable DNA would be of excellent value. A multiple linear regression model to predict the DNA content in incinerated pig teeth was developed based on the colourimetry, HA crystallite size and quantified nuclear and mitochondrial DNA. The chromaticity a* was found to be a significant predictor of the regression model. This study outlines a method to predict the viability of extracting nuclear and mitochondrial DNA from pig teeth that were exposed to a wide range of temperatures (27 to 1000 °C) with high accuracy (99.5–99.7%).

Similar content being viewed by others
References
Klempner S et al (2016) Processing skeletal samples for forensic DNA analysis. In: Katz E, Halámek J (eds) Forensic science: a multidisciplinary approach. John Wiley & Sons, Incorporated, Berlin, Germany, pp 177–192
Fredericks JD et al (2015) A potential new diagnostic tool to aid DNA analysis from heat compromised bone using colorimetry: a preliminary study. Sci Justice 55(2):124–130
Budowle B, Bieber FR, Eisenberg AJ (2005) Forensic aspects of mass disasters: strategic considerations for DNA-based human identification. Legal Med 7(4):230–243
Rennick SL, Fenton TW, Foran DR (2005) The effects of skeletal preparation techniques on DNA from human and non-human bone. J Forensic Sci 50(5):JFS2004405-4
Fredericks JD et al (2012) FTIR spectroscopy: a new diagnostic tool to aid DNA analysis from heated bone. Forensic Sci Int Genet 6(3):375–380
Haynes S et al (2002) Bone preservation and ancient DNA: the application of screening methods for predicting DNA survival. J Archaeol Sci 29(6):585–592
Kontopoulos I et al (2019) Petrous bone diagenesis: a multi-analytical approach. Palaeogeogr Palaeoclimatol Palaeoecol 518:143–154
Leskovar T et al (2020) ATR-FTIR spectroscopy combined with data manipulation as a pre-screening method to assess DNA preservation in skeletal remains. Forensic Sci Int Genet 44:102196
Schwarz C et al (2009) New insights from old bones: DNA preservation and degradation in permafrost preserved mammoth remains. Nucleic Acids Res 37(10):3215–3229
Wadsworth C et al (2017) Comparing ancient DNA survival and proteome content in 69 archaeological cattle tooth and bone samples from multiple European sites. J Proteome 158:1–8
Rubio L et al (2018) Dental color measurement to predict DNA concentration in incinerated teeth for human identification. PLoS One 13(4):e0196305
Sosa C et al (2013) Association between ancient bone preservation and DNA yield: A multidisciplinary approach. Am J Phys Anthropol 151(1):102–109
Higgins D et al (2013) Targeted sampling of cementum for recovery of nuclear DNA from human teeth and the impact of common decontamination measures. Investig Genet 4(1):18
Higgins D et al (2015) Differential nuclear and mitochondrial DNA preservation in post-mortem teeth with implications for forensic and ancient DNA studies. PLoS One 10(5):e0126935
Dobberstein RC et al (2008) Degradation of biomolecules in artificially and naturally aged teeth: implications for age estimation based on aspartic acid racemization and DNA analysis. Forensic Sci Int 179(2-3):181–191
Latham KE, Miller JJ (2019) DNA recovery and analysis from skeletal material in modern forensic contexts. Forensic Sci Res 4(1):51–59
De Boer HH et al (2018) DNA identification of human remains in Disaster Victim Identification (DVI): an efficient sampling method for muscle, bone, bone marrow and teeth. Forensic Sci Int 289:253–259
Biesecker LG et al (2005) DNA Identifications after the 9/11 World Trade Center Attack. Science 310(5751):1122–1123
Hartman D et al (2011) The contribution of DNA to the disaster victim identification (DVI) effort. Forensic Sci Int 205(1-3):52–58
Higgins D, Austin JJ (2013) Teeth as a source of DNA for forensic identification of human remains: A Review. Sci Justice 53(4):433–441
Robinson F, Rueggeberg F, Lockwood P (1998) Thermal stability of direct dental esthetic restorative materials at elevated temperatures. J Forensic Sci 43(6):1163–1167
Driessens FCM, Verbeeck RMH (1990) Biominerals. CRC Press, Boca Raton, p 428
Adler CJ et al (2011) Survival and recovery of DNA from ancient teeth and bones. J Archaeol Sci 38(5):956–964
Grunenwald A et al (2014) Adsorption of DNA on biomimetic apatites: Toward the understanding of the role of bone and tooth mineral on the preservation of ancient DNA. Appl Surf Sci 292:867–875
Brundin M et al (2013) DNA Binding to hydroxyapatite: a potential mechanism for preservation of microbial DNA. J Endod 39(2):211–216
Bernardi G (1965) Chromatography of nucleic acids on hydroxyapatite. Nature 206(4986):779–783
Tütken T, Vennemann TW (2011) Fossil bones and teeth: preservation or alteration of biogenic compositions? Palaeogeogr Palaeoclimatol Palaeoecol 310(1):1-8
Salamon M et al (2005) Relatively well preserved DNA is present in the crystal aggregates of fossil bones. Proc Natl Acad Sci U S A 102(39):13783
Alaeddini R, Walsh SJ, Abbas A (2010) Forensic implications of genetic analyses from degraded DNA—a review. Forensic Sci Int Genet 4(3):148–157
Hofreiter M et al (2001) Ancient DNA. Nat Rev Genet 2(5):353–359
Trueman CN, Martill DM (2002) The long–term survival of bone: the role of bioerosion. Archaeometry 44(3):371–382
Okazaki M et al (2001) Affinity binding phenomena of DNA onto apatite crystals. Biomaterials 22(18):2459–2464
del Valle LJ et al (2014) DNA adsorbed on hydroxyapatite surfaces. J Mater Chem B 2(40):6953–6966
Doebelin N, Kleeberg R (2015) Profex: a graphical user interfacefor the Rietveld refinement program BGMN. J Appl Crystallogr 48(Pt 5):1573–1580
Antinick TC, Foran DR (2019) Intra- and inter-element variability in mitochondrial and nuclear DNA from fresh and environmentally exposed skeletal remains. J Forensic Sci 64(1):88–97
R Core Team (2013) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria
Wei T et al (2017) Visualization of a correlation matrix, in Package “corrplot”
Rahmat RA et al (2020) Integrating spectrophotometric and XRD analyses in the investigation of burned dental remains. Forensic Sci Int 310:110–236
Rees KA, Cox MJ (2010) Comparative analysis of the effects of heat on the PCR-amplification of various sized DNA fragments extracted from Sus Scrofa molars. J Forensic Sci 55(2):410–417
Garriga JA, Ubelaker DH, Zapico SC (2016) Evaluation of macroscopic changes and the efficiency of DNA profiling from burnt teeth. Sci Justice
Thompson TJU (2005) Heat-induced dimensional changes in bone and their consequences for forensic anthropology. J Forensic Sci 50(5):1008–1015
Sakae T et al (1995) Thermal stability of mineralized and demineralized dentin: a differential scanning calorimetric study. Connect Tissue Res 33(1-3):193–196
Latham KE, Madonna ME (2013) DNA survivability in skeletal remains. In: Pokines J, Symes SA (eds) Manual of forensic taphonomy. CRC Press, Boca Raton, pp 385–407
Shipman P, Foster G, Schoeninger M (1984) Burnt bones and teeth: an experimental study of color, morphology, crystal structure and shrinkage. J Archaeol Sci 11(4):307–325
Sandholzer M (2014) Heat-induced alterations of dental tissues. ProQuest Dissertations Publishing
Kubisz L, Mielcarek S (2005) Differential scanning calorimetry and temperature dependence of electric conductivity in studies on denaturation process of bone collagen. J Non-Cryst Solids 351(33):2935–2939
Obal M et al (2019) Different skeletal elements as a source of DNA for genetic identification of Second World War victims. Forensic Sci Int Genet Suppl Ser 7(1):27–29
Alonso A et al (2004) Real-time PCR designs to estimate nuclear and mitochondrial DNA copy number in forensic and ancient DNA studies. Forensic Sci Int 139(2):141–149
Swango KL et al (2006) A quantitative PCR assay for the assessment of DNA degradation in forensic samples. Forensic Sci Int 158(1):14–26
Emery MV et al (2020) Reconstructing full and partial STR profiles from severely burned human remains using comparative ancient and forensic DNA extraction techniques. Forensic Sci Int Genet:46
Ricaut FX et al (2005) STR-genotyping from human medieval tooth and bone samples. Forensic Sci Int 151(1):31–35
Sobrino B, Brion M, Carracedo A (2005) SNPs in forensic genetics: a review on SNP typing methodologies. Forensic Sci Int:181
Budowle B, van Daal A (2008) Forensically relevant SNP classes. BioTechniques 44(5):603–610
Bender K, Schneider PM, Rittner C (2000) Application of mtDNA sequence analysis in forensic casework for the identification of human remains. Forensic Sci Int 113(1):103–107
Rubio L et al (2009) Study of short- and long-term storage of teeth and its influence on DNA. J Forensic Sci 54(6):1411–1413
Mundorff AZ, Bartelink EJ, Mar-Cash E (2009) DNA preservation in skeletal elements from the World Trade Center disaster: recommendations for mass fatality management. J Forensic Sci 54(4):739–745
Robinson C et al (1988) Mineral and protein concentrations in enamel of the developing permanent porcine dentition. Caries Res 22(6):321–326
Piga G et al (2013) Is X-ray diffraction able to distinguish between animal and human bones? J Archaeol Sci 40(1):778–785
Matuszewski S et al (2020) Pigs vs people: the use of pigs as analogues for humans in forensic entomology and taphonomy research. Int J Legal Med 134:793–810
Acknowledgements
The main author would like to acknowledge Associate Professor Jeremy Austin and the Advanced DNA Identification & Forensic Facility (ADIFF), The University of Adelaide, for providing supervision, laboratory space, and equipment to conduct the DNA work.
Sincere gratitude to Professor Robert Fitzpatrick and Mr. Mark Raven from the Acid Sulfate Soils Centre (ASSC) | Centre for Australian Forensic Soil Science (CAFSS), The University of Adelaide, for providing expertise, facility and equipment for the sample incineration and XRD analysis at Water and Land, CSIRO, Waite, South Australia. The first author would like to give a special thanks to Mr. Anthony Wilkes from the School of Animal and Veterinary Sciences, The University of Adelaide, for providing the technical assistance in preparing the samples.
Funding
This work was financially supported by the School of Biological Sciences, The University of Adelaide.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
The handling of the animal remains was conducted according to the University of Adelaide Animal Ethics.
Research involving human participants and/or animals
This research involved animal carcasses.
Informed consent
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(DOCX 553 kb)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Rahmat, R.A., Humphries, M.A., Saedon, N.A. et al. Diagnostic models to predict nuclear DNA and mitochondrial DNA recovery from incinerated teeth. Int J Legal Med 137, 1353–1360 (2023). https://doi.org/10.1007/s00414-023-03017-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00414-023-03017-x