Abstract
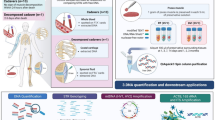
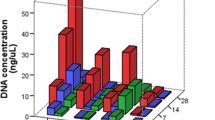
Filter papers have been used for many years in different applications of molecular biology and have been proven to be a stable way to store DNA waiting to be analyzed. Sampling of DNA on FTA (Flinders Technology Associates) cards is convenient and cost effective compared to alternative approaches involving DNA extractions and storage of DNA extracts. FTA cards are analyzed at many forensic laboratories, and the way to perform direct genetic profiling on buccal swab cards has developed into an almost industrial process. The possibility to include postmortem (PM) samples into an FTA-based workflow would facilitate and speed up the genetic identification process compared to conventional methods, both on a regular basis and in a mass casualty event. In this study, we investigated if FTA cards may be used to carry tissue DNA from deceased and present a high-quality DNA profile from the individual in order to be useful for the identification process. The study also aimed to investigate if a specific body tissue would be preferable, and if decomposed tissue is suitable at all to put on an FTA card in order to obtain a DNA profile. We have compared the quality of the DNA profiles acquired from postmortem tissue on FTA cards, with the results acquired with conventional methods from reference bone/muscle samples from the same individual. Several types of tissues have been tested from different identification cases and scenarios. We concluded that tissue cells from inner organs are suitable to put on FTA cards, and that the obtained DNA profiles have the potential to serve as PM data for identification purposes. In cases including compromised samples, however, it is recommended to keep the tissue sample as a backup if further DNA has to be extracted.



Similar content being viewed by others
References
Luckwell J, Denniff P, Capper S, Michael P, Spooner N, Mallender P, Johnson B, Clegg S, Green M, Ahmad S, Woodford L (2013) Assessment of the within- and between-lot variability of Whatman™ FTA(®) DMPK and 903(®) DBS papers and their suitability for the quantitative bioanalysis of small molecules. Bioanalysis 5(21):2613–2630
Liang X, Chigerwe M, Hietala SK, Crossley BM (2014) Evaluation of fast technology analysis (FTA) cards as an improved method for specimen collection and shipment targeting viruses associated with bovine respiratory disease complex. J Virol Methods 202:69–72
Yee EY, Zainuddin ZZ, Ismail A, Yap CK, Tan SG (2013) Identification of hybrids of painted milky storks using FTA card-collected blood, molecular markers and morphologies. Biochem Genet 51(9–10):789–799
Aye KS, Matsouka M, Kai M, Kyaw K, Win AA, Shwe MM, Thein M, Htoo MM, Htoon MT (2011) FTA card utility for PCR detection of Mycobacterium leprae. Jpn J Infect Dis 64(3):246–248
Braae UC, Johansen MV, Ngowi HA, Rasmussen TB, Nielsen J, Uttenthal Å (2015) Detection of African swine fever virus DNA in blood samples stored on FTA cards from asymptomatic pigs in Mbeya region, Tanzania. Transbound Emerg Dis 62(1):87–90
Mohammadi R, Mirhendi H, Rezaei-Matehkolaei A, Ghahri M, Shidfar MR, Jalalizand N, Makimura K (2013) Molecular identification and distribution profile of Candida species isolated from Iranian patients. Med Mykol 51(6):657–663
Dobbs LJ, Madigan MN, Carter AB, Earls L (2002) Use of FTA gene guard filter paper for the storage and transportation of tumor cells for molecular testing. Arch Pathol Lab Med 126(1):56–63
Mundorff AZ, Amory S, Huel R, Bilic R, Scott AL, Parsons TJ (2018) An economical and efficient method for postmortem DNA sampling in mass fatalities. For Sci Int Genet 36:167–175
Dawnay N, Flamson R, Hall MJR, Steadman DW (2018) Impact of sample degradation and inhibition on field-based DNA identification of human remains. For Sci Int Genet 37:46–53
Winskog C, Nilsson H, Montelius K, Lindblom B (2010) The use of commercial alcohol products to sterilize bones prior to DNA sampling. Forensic Sci Med Pathol 6(2):127–129. https://doi.org/10.1007/s12024-010-9155-z
Montelius K, Karlsson AO, Holmlund G (2008) STR data for the AmpFlSTR Identifiler loci from Swedish population in comparison to European, as well as with non-European population. Forensic Sci Int Genet 2(3):e49–e52. https://doi.org/10.1016/j.fsigen.2007.12.005
ENSFI guide-lines (2010) Recommended minimum criteria for the validation of various aspects of the DNA profiling process policy ref. ENFSI DNA Working Group, Issue no: 001
Rahikainen A-L, Palo JU, de Leeuw W, Budowle B, Sajantila A (2016) DNA quality and quantity from up to 16 years old post-mortem blood stored on FTA cards. Forensic Sci Int 261:148–153. https://doi.org/10.1016/j.forsciint.2016.02.014. Epub2016Feb 23
Kline MC, Duewer DL, Redman JW, Butler JM, Boyer DA (2002) Polymerase chain reaction amplification of DNA from aged blood stains: quantitative evaluation of the “suitability for purpose” of four filter papers as archival media. Anal Chem 74(8):1863–1869
Mas S, Crescenti A, Gassó P, Vidal-Taboada JM, Lafuente A (2007) DNA cards: determinants of DNA yield and quality in collecting genetic samples for pharmacogenetic studies. Basic Clin Pharmacol Toxicol 101(2):132–137
Wilhelm AJ, den Burger JC, Swart EL (2014) Therapeutic drug monitoring by dried blood spot: progress to date and future directions. Clin Pharmacokinet 53(11):961–973. https://doi.org/10.1007/s40262-014-0177-7
Steinlechner M, Parson W, Rabl W, Grubwieser P, Scheithauer R (2005) TSUNAMI-Disaster: DNA typing of Sri Lanka victim samples and related AM matching procedures. Abstract ISFG 21st congress
Tack LC, Thomas M, Reich K (2007) Automated forensic DNA purification optimized for FTA card punches and identifiler STR-based PCR analysis. Clin Lab Med 27(1):183–191
Stangegaard M, Froeslev TG, Frank-Hansen R, Hansen AJ, Morling N (2011) Automated extraction of DNA from blood and PCR setup using a Tecan freedom EVO liquid handler for forensic genetic STR typing of reference samples. J Lab Autom 16(2):134–140. https://doi.org/10.1016/j.jala.2010.11.003
Acknowledgements
This research was conducted within the Strategic Research Area Forensic Sciences (Strategiområdet Forensiska Vetenskaper) at Linköping University.
This work was supported by National Board of Forensic Medicine.
This work was approved according to the National Board of Forensic Medicine research policy (RMV-policy No. 2008-01).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Green, H., Tillmar, A., Pettersson, G. et al. The use of FTA cards to acquire DNA profiles from postmortem cases. Int J Legal Med 133, 1651–1657 (2019). https://doi.org/10.1007/s00414-019-02015-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00414-019-02015-2