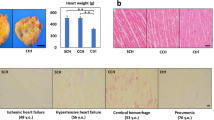
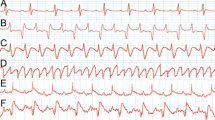
Abstract
To determine the cause of death in myocardial ion channel diseases (MICD)-induced sudden cardiac death (SCD) cases is a difficulty in forensic identification practices. The majority of MICD-induced SCD cases would experience lethal ventricular tachyarrhythmia (LVTA) before deaths; thus, confirming the occurrence of LVTA in bodies can offer a key evidence to identify these cases. Several lipids in the myocardia were found disturbed after LVTA; yet, whether serum lipidome would be disrupted by LVTA is not clear. Therefore, we aimed to screen lipid feature and related diagnostic markers of LVTA in serum here. An aconitine-induced LVTA-SCD rat model was produced. Blood samples before LVTA and immediately after LVTA were retrieved and related serum specimens were used for ultra-performance liquid chromatography-mass spectrometry (UPLC-MS)-based lipidomics analyses. On the basis of the defined differential lipids, a lipid-related metabolic pathway network was constructed and potential biomarkers were screened. Twelve aconitine-induced LVTA rats were produced. Totally, 188 lipids in serum were disrupted during the LVTA-SCD process, which belong to 11 lipid classes. Most of the differential lipids were correlated, suggesting that they were interacted and that the changes were holistic during LVTA process. Ten lipid pathways were activated during LVTA process; the main lipid classes involved in these pathways were ceramide, sphingomyelin, phosphatidylcholine, phosphatidylethanolamine, and phosphatidylserine. Phosphatidylcholine O-40:4, sphingomyelin d46:5, and phosphatidylethanolamine 40:4 were tested as potential diagnostic markers of LVTA-SCD event in serum. The current results indicate a substantial change in serum lipidome after LVTA-SCD; lipidomics holds promise to identify MICD-induced SCDs in forensic practices.






Similar content being viewed by others
References
Marsman RF, Tan HL, Bezzina CR (2014) Genetics of sudden cardiac death caused by ventricular arrhythmias. Nat Rev Cardiol 11(2):96–111. https://doi.org/10.1038/nrcardio.2013.186
Winkel BG, Holst AG, Theilade J, Kristensen IB, Thomsen JL, Ottesen GL, Bundgaard H, Svendsen JH, Haunso S, Tfelt-Hansen J (2011) Nationwide study of sudden cardiac death in persons aged 1-35 years. Eur Heart J 32(8):983–990. https://doi.org/10.1093/eurheartj/ehq428
Zipes DP, Wellens HJ (1998) Sudden cardiac death. Circulation 98(21):2334–2351
Ifteni P, Barabas B, Gavris C, Moga M, Burtea V, Dracea L (2017) Sudden cardiac death: autopsy findings in 7200 cases between 2001 and 2015. Am J Forensic Med Pathol 38(1):49–53. https://doi.org/10.1097/paf.0000000000000274
Basso C, Carturan E, Pilichou K, Rizzo S, Corrado D, Thiene G (2010) Sudden cardiac death with normal heart: molecular autopsy. Cardiovasc Pathol 19(6):321–325. https://doi.org/10.1016/j.carpath.2010.02.003
Corrado D, Basso C, Thiene G (2001) Sudden cardiac death in young people with apparently normal heart. Cardiovasc Res 50(2):399–408
Brion M, Sobrino B, Martinez M, Blanco-Verea A, Carracedo A (2015) Massive parallel sequencing applied to the molecular autopsy in sudden cardiac death in the young. Forensic Sci Int Genet 18:160–170. https://doi.org/10.1016/j.fsigen.2015.07.010
Chopra N, Knollmann BC (2011) Genetics of sudden cardiac death syndromes. Curr Opin Cardiol 26(3):196–203. https://doi.org/10.1097/HCO.0b013e3283459893
Wang X, Wang D, Yu X, Zhang G, Wu J, Zhu G, Su R, Lv J (2016) Non-targeted metabolomics identified a common metabolic signature of lethal ventricular tachyarrhythmia (LVTA) in two rat models. Mol BioSyst 12(7):2213–2223. https://doi.org/10.1039/c6mb00080k
Curtis MJ, Walker MJ (1988) Quantification of arrhythmias using scoring systems: an examination of seven scores in an in vivo model of regional myocardial ischaemia. Cardiovasc Res 22(9):656–665
Gibellini F, Smith TK (2010) The Kennedy pathway—de novo synthesis of phosphatidylethanolamine and phosphatidylcholine. IUBMB Life 62(6):414–428. https://doi.org/10.1002/iub.337
An Nguyen SAR, Zhang Q, Michael JO Wakelam Using lipidomics analysis to determine signalling and metabolic changes in cells
Bach D, Epand RF, Epand RM, Wachtel E (2008) Interaction of 7-ketocholesterol with two major components of the inner leaflet of the plasma membrane: phosphatidylethanolamine and phosphatidylserine. Biochemistry 47(9):3004–3012. https://doi.org/10.1021/bi702070b
Sun GB, Sun H, Meng XB, Hu J, Zhang Q, Liu B, Wang M, HB X, Sun XB (2014) Aconitine-induced Ca2+ overload causes arrhythmia and triggers apoptosis through p38 MAPK signaling pathway in rats. Toxicol Appl Pharmacol 279(1):8–22. https://doi.org/10.1016/j.taap.2014.05.005
Zimmer T, Surber R (2008) SCN5A channelopathies—an update on mutations and mechanisms. Prog Biophys Mol Biol 98(2–3):120–136. https://doi.org/10.1016/j.pbiomolbio.2008.10.005
Liu N, Denegri M, Dun W, Boncompagni S, Lodola F, Protasi F, Napolitano C, Boyden PA, Priori SG (2013) Abnormal propagation of calcium waves and ultrastructural remodeling in recessive catecholaminergic polymorphic ventricular tachycardia. Circ Res 113(2):142–152. https://doi.org/10.1161/circresaha.113.301783
Bezzina CR, Lahrouchi N, Priori SG (2015) Genetics of sudden cardiac death. Circ Res 116(12):1919–1936. https://doi.org/10.1161/CIRCRESAHA.116.304030
Guarino MP, Santos AI, Mota-Carmo M, Costa PF (2013) Effects of anaesthesia on insulin sensitivity and metabolic parameters in Wistar rats. In Vivo (Athens, Greece) 27(1):127–132
Das G, Vernunft A, Gors S, Kanitz E, Weitzel JM, Brussow KP, Metges CC (2016) Acute effects of general anesthesia with propofol, pentobarbital or isoflurane plus propofol on plasma metabolites and hormones in adult pigs. J Anim Sci 94(12):5182–5191. https://doi.org/10.2527/jas.2016-1018
Hirvonen J, Kortelainen ML, Huttunen P (1997) Pulmonary and serum surfactant phospholipids and serum catecholamines in strangulation. An experimental study on rats. Forensic Sci Int 90(1–2):17–24
Wang Q, Ishikawa T, Michiue T, Zhu BL, Guan DW, Maeda H (2012) Intrapulmonary aquaporin-5 expression as a possible biomarker for discriminating smothering and choking from sudden cardiac death: a pilot study. Forensic Sci Int 220(1–3):154–157. https://doi.org/10.1016/j.forsciint.2012.02.013
Verkleij AJ, Leunissen-Bijvelt J, de Kruijff B, Hope M, Cullis PR (1984) Non-bilayer structures in membrane fusion. CIBA Found Symp 103:45–59
Wang Z, Klipfell E, Bennett BJ, Koeth R, Levison BS, Dugar B, Feldstein AE, Britt EB, Fu X, Chung YM, Wu Y, Schauer P, Smith JD, Allayee H, Tang WH, DiDonato JA, Lusis AJ, Hazen SL (2011) Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature 472(7341):57–63. https://doi.org/10.1038/nature09922
Stegemann C, Pechlaner R, Willeit P, Langley SR, Mangino M, Mayr U, Menni C, Moayyeri A, Santer P, Rungger G, Spector TD, Willeit J, Kiechl S, Mayr M (2014) Lipidomics profiling and risk of cardiovascular disease in the prospective population-based Bruneck study. Circulation 129(18):1821–1831. https://doi.org/10.1161/CIRCULATIONAHA.113.002500
Hatch GM, O K CPC (1989) Regulation of phosphatidylcholine metabolism in mammalian hearts. Biochem Cell Biol 67(2–3):67–77
Chen X, Sun A, Zou Y, Ge J, Lazar JM, Jiang XC (2011) Impact of sphingomyelin levels on coronary heart disease and left ventricular systolic function in humans. Nutr Metab 8(1):25. https://doi.org/10.1186/1743-7075-8-25
Chapman H, Ramstrom C, Korhonen L, Laine M, Wann KT, Lindholm D, Pasternack M, Tornquist K (2005) Downregulation of the HERG (KCNH2) K(+) channel by ceramide: evidence for ubiquitin-mediated lysosomal degradation. J Cell Sci 118(Pt 22):5325–5334. https://doi.org/10.1242/jcs.02635
Park TS, Hu Y, Noh HL, Drosatos K, Okajima K, Buchanan J, Tuinei J, Homma S, Jiang XC, Abel ED, Goldberg IJ (2008) Ceramide is a cardiotoxin in lipotoxic cardiomyopathy. J Lipid Res 49(10):2101–2112. https://doi.org/10.1194/jlr.M800147-JLR200
Zhou YT, Grayburn P, Karim A, Shimabukuro M, Higa M, Baetens D, Orci L, Unger RH (2000) Lipotoxic heart disease in obese rats: implications for human obesity. Proc Natl Acad Sci U S A 97(4):1784–1789
Chugh SS, Kelly KL, Titus JL (2000) Sudden cardiac death with apparently normal heart. Circulation 102(6):649–654
Funding
This work was supported by grants from the Natural Science Foundation (2015A408119346049), the Science and Technology Innovation project (2013KJCX0076) of Guangdong Province, and the Natural Science Foundation of Shanghai (15ZR1430300), China.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This study was approved by the Medical Animal Care and Welfare Committee at Shantou University Medical College (number of authorization: SUMC 2014-157). All procedures were carried out in accordance with the Guide for Care and Use of Laboratory Animals of our College.
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
ESM 1
(DOCX 15749 kb)
Rights and permissions
About this article
Cite this article
Wu, J., Wu, Q., Dai, W. et al. Serum lipid feature and potential biomarkers of lethal ventricular tachyarrhythmia (LVTA) induced by myocardial ion channel diseases: a rat model study. Int J Legal Med 132, 439–448 (2018). https://doi.org/10.1007/s00414-017-1710-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00414-017-1710-7