Abstract
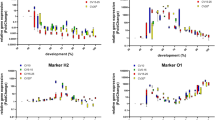
The analysis of insect evidence is often used in death investigations as the development of necrophagous insect species can be used to determine a minimum post-mortem interval (PMImin). Usually, the PMImin estimations are based on the age estimation of larvae developed on the corpse. So far, age estimation mostly relies on length or weight measurement of the larvae. These measurements are then compared to species-specific reference growth data obtained from laboratory studies. However, length and weight do not always represent the best developmental markers to provide accurate and precise age estimates for PMImin calculation, especially for post-feeding third-instar larvae which decrease in size. Therefore, more growth markers are required to improve age estimation not only for post-feeding larvae but also for all larval stages. As the analysis of temporally regulated genes proved suitable for age prediction in blow fly pupae, we examined the gene expression patterns of six genes (15_2, 2014192, EcdR, AR, hsp90 and actin) during larval development of Calliphora vicina at three constant temperatures and analysed the effect of a combination of morphological and molecular age markers on statistical models of development. EcdR, AR and 15_2 showed reliable tendencies to classify the post-feeding stage more precisely, and inclusion of gene expression data in models of development improved the statistical fit of the model. Nevertheless, for depicting the first larval stages and for quantifying the onset of the post-feeding stage more accurately, results of this preliminary study should be supported by searching for more informative genes.








Similar content being viewed by others
References
Amendt J, Zehner R, Krettek R (2004) Forensic entomology. Naturwissenschaften 91:51–65
Catts EP, Goff ML (1992) Forensic entomology in criminal investigations. Annu Rev Entomol 37:253–272
Tomberlin JK, Mohr R, Benbow ME, Tarone AM, VanLaerhoven S (2011) A roadmap for bridging basic and applied research in forensic entomology. Annu Rev Entomol 56:401–421
Fiene JG, Sword GA, Van Laerhoven SL, Tarone AM (2014) The role of spatial aggregation in forensic entomology. J Med Entomol 51:1–9
Byrd JJ, Castner JL (2001) The utility of arthropods in legal investigations. CRC Press Boca Raton, FL
Campobasso CP, Di Vella G, Introna F (2001) Factors affecting decomposition and Diptera colonization. Forensic Sci Int 120:18–27
Goff ML (2001) A fly for the prosecution—how insect evidence helps solve crimes. Harvard University Press, Cambridge
Greenberg B (1991) Flies as forensic indicators. J Med Entomol 28:565–577
Wells J, LaMotte LR (1995) Estimating maggot age from weight using inverse prediction. J Forensic Sci 40:585–590
Higley LG, Haskell NH (2001) Insect development and forensic entomology. In: Byrd JJ, Castner JL (eds) The utility of arthropods in legal investigations. CRC Press, Boca Raton, pp 287–300
Reiter C (1984) Zum Wachstumsverhalten der Maden der blauen Schmeißfliege Calliphora vicina. Z Rechtsmed 91:295–308
Grassberger M, Reiter C (2001) Effect of temperature on Lucilia sericata (Diptera: Calliphoridae) development with special references to the isomegalen- and isomorphen-diagram. Forensic Sci Int 120:32–36
Richards CS, Crous KL, Villet MH (2009) Models of development for blowfly sister species Chrysomya chloropyga and Chrysomya putoria. Med Vet Entomol 23:56–61
Amendt J, Campobasse CP, Gaudry E, Reiter C, LeBlanc HN, Hall M (2007) Best practise in forensic entomology—standards and guidelines. Int J Legal Med 121:90–104
Anderson GS (2004) Determining time of death using blow fly eggs in the early post-mortem interval. Int J Legal Med 118:240–241
Baqué M, Filmann N, Verhoff MA, Amendt J (2014) Establishment of developmental charts for the larvae of the blow fly Calliphora vicina using quantile regression. Forensic Sci Int. doi:10.1016/j.forsciint.2014.12.020
Tarone AM, Foran DR (2011) Gene expression during blow fly development: improving the precision of age estimates in forensic entomology. J Forensic Sci 56:112–122
Marchenko MI (2001) Medicolegal relevance of cadaver entomofauna for the determination of the time of death. Forensic Sci Int 120:89–109
Boehme P, Spahn P, Amendt J, Zehner R (2013) Differential gene expression during metamorphosis: a promising approach for age estimation of forensically important Calliphora vicina pupae (Diptera: Calliphoridae). Int J Legal Med 127:243–249
Boehme P, Spahn P, Amendt J, Zehner R (2014) The analysis of temporal gene expression to estimate the age of forensically important blow fly pupae: results from three blind studies. Int J Legal Med 128:565–573
Fremdt H, Amendt J, Zehner R (2013) Diapause-specific gene expression in Calliphora vicina (Diptera: Calliphoridae)—a useful diagnostic tool for forensic entomology. Int J Legal Med. doi:10.1007/s00414-013-0920-x
Tarone AM, Jennings KC, Foran DR (2007) Aging blow fly eggs using gene expression: a feasibility study. J Forensic Sci 52:1350–1354
Brown K, Thorne A, Harvey M (2014) Calliphora vicina (Diptera: Calliphoridae) pupae: a timeline of external morphological development and a new age and PMI estimation tool. Int J Legal Med. doi:10.1007/s00414-014-1068-z
Davies K, Harvey ML (2013) Internal morphological analysis for age estimation of blow fly pupae (Diptera: Calliphoridae) in postmortem interval estimation. J Forensic Sci 58:79–84
Zajac BK, Amendt J (2012) Bestimmung des Alters forensisch relevanter Fliegenpuppen. Rechtsmedizin 22:456–465
Zehner R, Mösch S, Amendt J (2006) Estimating the postmortem interval by determining the age of fly pupae: are there any molecular tools? Prog Forensic Genet 11:619–621, ICS 1288
Michaud J-P, Schoenly KG, Moreau G (2012) Sampling flies or sampling flaws? Experimental design and inference strength in forensic entomology. J Med Entomol 49:1–10
Villet MH (2007) An inexpensive geometrical micrometer for measuring small, live insects quickly without harming them. Entomol Exp Appl 122:279–280
Prins AJ (1982) Morphological and biological notes on six South African blow-flies (Diptera, Calliphoridae) and their immature stages. Ann South Afr Mus 90:201–217
Bustin SA, Benes V, Garson JA, Hellemans J, Huggett J, Kubista M, Mueller R, Nolan T, Pfaffl MW, Shipley GL, Vandesompele J, Wittwer CT (2009) The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin Chem 55:611–622
Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F (2002) Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol 3:research0034.1–research0034.11
Zuur AF, Ieno EN, Smith GM (2007) Analysing ecological data. Springer, New York
Ieno EN, Amendt J, Fremdt H, Saveliev AA, Zuur AF (2010) Analysing forensic entomology data using additive mixed effects modelling. In: Amendt J, Goff Ml, Campobasso C, Grassberger M (eds) Current. Springer, pp. 139-162
R Core Team (2014) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, http://www.R-project.org/
Quinn GP, Keough MJ (2002) Experimental design and data analysis for biologists. Cambridge University Press, Cambridge
Baqué M, Amendt J (2013) Strengthen forensic entomology in court—the need for data exploration and the validation of a generalised additive mixed model. Int J Legal Med 127:213–223
Ames C, Turner B, Daniel B (2006) Estimating the post-mortem interval (II): the use of differential temporal gene expression to determine the age of blowfly pupae. Int Congr Ser 1288:861–863
Burmester T, Scheller K (1995) Complete cDNA-sequence of the receptor responsible for arylphorin uptake by the larval fat body of the blowfly, Calliphora vicina. Insect Biochem Mol 25:981–989
Buszczak M, Segraves WA (2000) Insect metamorphosis: out with the old, in with the new. Curr Biol 10:R380–R833
Koelle MR, Talbot WS, Segraves WA, Bender MT, Cherbas P, Hogness DS (1991) The Drosophila EcR gene encodes an ecdysone receptor, a new member of the steroid receptor superfamily. Cell 4:59–77
Burmester T, Scheller K (1997) Developmentally controlled cleavage of the Calliphora arylphorin receptor and posttranslational action of the steroid hormone 20-hydroxyecdysone. Eur J Biochem 247:1062–1068
Zajac BK, Amendt J, Horres R, Verhoff MA, Zehner R (2014) De novo transcriptome analysis and highly sensitive digital gene expression profiling of Calliphora vicina (Diptera: Calliphoridae) pupae using MACE (massive analysis of cDNA ends). Forensic Sci Int: Genet. doi:10.1016/j.fsigen.2014.11.013
Zuur AF, Ieno EN, Walker N, Saveliev AA, Smith GM (2009) Mixed effects models and extensions in ecology with R. Springer, New York
Acknowledgments
We thank Barbara K. Zajac, Petra Böhme and the anonymous reviewers for correcting the language of the manuscript and their helpful comments and suggestions.
Compliance with ethical standards
Disclosure of potential conflicts of interest
Conflict of interest
The authors declare that they have no conflict of interest.
Research involving human participants and/or animals
Ethical approval
All applicable international, national and/or institutional guidelines for the care and use of animals were followed. This article does not contain any studies with human participants performed by any of the authors.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 44 kb)
Rights and permissions
About this article
Cite this article
Baqué, M., Amendt, J., Verhoff, M.A. et al. Descriptive analyses of differentially expressed genes during larval development of Calliphora vicina (Diptera: Calliphoridae). Int J Legal Med 129, 891–902 (2015). https://doi.org/10.1007/s00414-015-1180-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00414-015-1180-8