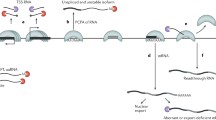
Abstract
Formation of functional mRNA–protein particles requires a plethora of nuclear cotranscriptional and posttranscriptional RNA processing and packaging steps. Faithful execution of these events is closely monitored by surveillance systems that prevent nuclear export of, and/or rapidly degrade, faulty transcripts. Parts of this quality control also serve to eliminate a large number of noncoding RNAs produced by RNA polymerase II. Here, we discuss which aberrant features trigger messenger ribonucleoprotein quality control, how the process is executed, and how it is connected to the transcription machinery and the nuclear pore complex.



Similar content being viewed by others
References
Abruzzi KC, Lacadie S, Rosbash M (2004) Biochemical analysis of TREX complex recruitment to intronless and intron-containing yeast genes. Embo J 23:2620–2631
Abruzzi KC, Belostotsky DA, Chekanova JA, Dower K, Rosbash M (2006) 3′-end formation signals modulate the association of genes with the nuclear periphery as well as mRNP dot formation. Embo J 25:4253–4262
Adamson TE, Shutt DC, Price DH (2005) Functional coupling of cleavage and polyadenylation with transcription of mRNA. J Biol Chem 280:32262–32271
Allmang C, Kufel J, Chanfreau G, Mitchell P, Petfalski E, Tollervey D (1999a) Functions of the exosome in rRNA, snoRNA and snRNA synthesis. Embo J 18:5399–5410
Allmang C, Petfalski E, Podtelejnikov A, Mann M, Tollervey D, Mitchell P (1999b) The yeast exosome and human PM-Scl are related complexes of 3′ –> 5′ exonucleases. Genes Dev 13:2148–2158
Andrulis ED, Werner J, Nazarian A, Erdjument-Bromage H, Tempst P, Lis JT (2002) The RNA processing exosome is linked to elongating RNA polymerase II in Drosophila. Nature 420:837–841
Arigo JT, Carroll KL, Ames JM, Corden JL (2006a) Regulation of yeast NRD1 expression by premature transcription termination. Mol Cell 21:641–651
Arigo JT, Eyler DE, Carroll KL, Corden JL (2006b) Termination of cryptic unstable transcripts is directed by yeast RNA-binding proteins Nrd1 and Nab3. Mol Cell 23:841–851
Bentley DL (2005) Rules of engagement: co-transcriptional recruitment of pre-mRNA processing factors. Curr Opin Cell Biol 17:251–256
Bird G, Fong N, Gatlin JC, Farabaugh S, Bentley DL (2005) Ribozyme cleavage reveals connections between mRNA release from the site of transcription and pre-mRNA processing. Mol Cell 20:747–758
Bousquet-Antonelli C, Presutti C, Tollervey D (2000) Identification of a regulated pathway for nuclear pre-mRNA turnover. Cell 102:765–775
Brickner DG, Cajigas I, Fondufe-Mittendorf Y, Ahmed S, Lee PC, Widom J, Brickner JH (2007) H2A.Z-mediated localization of genes at the nuclear periphery confers epigenetic memory of previous transcriptional state. PLoS Biol 5:e81
Briggs MW, Burkard KT, Butler JS (1998) Rrp6p, the yeast homologue of the human PM-Scl 100-kDa autoantigen, is essential for efficient 5.8 S rRNA 3′ end formation. J Biol Chem 273:13255–13263
Burgess SM, Guthrie C (1993) A mechanism to enhance mRNA splicing fidelity: the RNA-dependent ATPase Prp16 governs usage of a discard pathway for aberrant lariat intermediates. Cell 73:1377–1391
Burkard KT, Butler JS (2000) A nuclear 3′-5′ exonuclease involved in mRNA degradation interacts with Poly(A) polymerase and the hnRNA protein Npl3p. Mol Cell Biol 20:604–616
Canavan R, Bond U (2007) Deletion of the nuclear exosome component RRP6 leads to continued accumulation of the histone mRNA HTB1 in S-phase of the cell cycle in Saccharomyces cerevisiae. Nucleic Acids Res 35:6268–6279
Carroll KL, Pradhan DA, Granek JA, Clarke ND, Corden JL (2004) Identification of cis elements directing termination of yeast nonpolyadenylated snoRNA transcripts. Mol Cell Biol 24:6241–6252
Carroll KL, Ghirlando R, Ames JM, Corden JL (2007) Interaction of yeast RNA-binding proteins Nrd1 and Nab3 with RNA polymerase II terminator elements. Rna 13:361–373
Casolari JM, Brown CR, Komili S, West J, Hieronymus H, Silver PA (2004) Genome-wide localization of the nuclear transport machinery couples transcriptional status and nuclear organization. Cell 117:427–439
Chavez S, Beilharz T, Rondon AG, Erdjument-Bromage H, Tempst P, Svejstrup JQ, Lithgow T, Aguilera A (2000) A protein complex containing Tho2, Hpr1, Mft1 and a novel protein, Thp2, connects transcription elongation with mitotic recombination in Saccharomyces cerevisiae. Embo J 19:5824–5834
Chekanova JA, Gregory BD, Reverdatto SV, Chen H, Kumar R, Hooker T, Yazaki J, Li P, Skiba N, Peng Q, Alonso J, Brukhin V, Grossniklaus U, Ecker JR, Belostotsky DA (2007) Genome-wide high-resolution mapping of exosome substrates reveals hidden features in the Arabidopsis transcriptome. Cell 131:1340–1353
Chekanova JA, Abruzzi KC, Rosbash M, Belostotsky DA (2008) Sus1, Sac3, and Thp1 mediate post-transcriptional tethering of active genes to the nuclear rim as well as to non-nascent mRNP. Rna 14:66–77
Custodio N, Carmo-Fonseca M, Geraghty F, Pereira HS, Grosveld F, Antoniou M (1999) Inefficient processing impairs release of RNA from the site of transcription. Embo J 18:2855–2866
Custodio N, Vivo M, Antoniou M, Carmo-Fonseca M (2007) Splicing- and cleavage-independent requirement of RNA polymerase II CTD for mRNA release from the transcription site. J Cell Biol 179:199–207
Damgaard CK, Kahns S, Lykke-Andersen S, Nielsen AL, Jensen TH, Kjems J (2008) A 5′ splice site enhances the recruitment of basal transcription initiation factors in vivo. Mol Cell 29:271–278
Danin-Kreiselman M, Lee CY, Chanfreau G (2003) RNAse III-mediated degradation of unspliced pre-mRNAs and lariat introns. Mol Cell 11:1279–1289
Das B, Butler JS, Sherman F (2003) Degradation of normal mRNA in the nucleus of Saccharomyces cerevisiae. Mol Cell Biol 23:5502–5515
Dower K, Kuperwasser N, Merrikh H, Rosbash M (2004) A synthetic A tail rescues yeast nuclear accumulation of a ribozyme-terminated transcript. Rna 10:1888–1899
Dziembowski A, Ventura AP, Rutz B, Caspary F, Faux C, Halgand F, Laprevote O, Seraphin B (2004) Proteomic analysis identifies a new complex required for nuclear pre-mRNA retention and splicing. Embo J 23:4847–4856
Fischer T, Strasser K, Racz A, Rodriguez-Navarro S, Oppizzi M, Ihrig P, Lechner J, Hurt E (2002) The mRNA export machinery requires the novel Sac3p–Thp1p complex to dock at the nucleoplasmic entrance of the nuclear pores. Embo J 21:5843–5852
Furger A, O’Sullivan JM, Binnie A, Lee BA, Proudfoot NJ (2002) Promoter proximal splice sites enhance transcription. Genes Dev 16:2792–2799
Galy V, Gadal O, Fromont-Racine M, Romano A, Jacquier A, Nehrbass U (2004) Nuclear retention of unspliced mRNAs in yeast is mediated by perinuclear Mlp1. Cell 116:63–73
Giaever G, Flaherty P, Kumm J, Proctor M, Nislow C, Jaramillo DF, Chu AM, Jordan MI, Arkin AP, Davis RW (2004) Chemogenomic profiling: identifying the functional interactions of small molecules in yeast. Proc Natl Acad Sci U S A 101:793–798
Guo X, Ma J, Sun J, Gao G (2007) The zinc-finger antiviral protein recruits the RNA processing exosome to degrade the target mRNA. PNAS 104:151–156
Hieronymus H, Yu MC, Silver PA (2004) Genome-wide mRNA surveillance is coupled to mRNA export. Genes Dev 18:2652–2662
Hilleren PJ, Parker R (2003) Cytoplasmic degradation of splice-defective pre-mRNAs and intermediates. Mol Cell 12:1453–1465
Hilleren P, McCarthy T, Rosbash M, Parker R, Jensen TH (2001) Quality control of mRNA 3′-end processing is linked to the nuclear exosome. Nature 413:538–542
Isken O, Maquat LE (2007) Quality control of eukaryotic mRNA: safeguarding cells from abnormal mRNA function. Genes Dev 21:1833–1856
Iwanejko L, Smith KN, Loeillet S, Nicolas A, Fabre F (1999) Disruption and functional analysis of six ORFs on chromosome XV: YOL117w, YOL115w (TRF4), YOL114c, YOL112w (MSB4), YOL111c and YOL072w. Yeast 15:1529–1539
Jensen TH, Boulay J, Rosbash M, Libri D (2001a) The DECD box putative ATPase Sub2p is an early mRNA export factor. Curr Biol 11:1711–1715
Jensen TH, Patricio K, McCarthy T, Rosbash M (2001b) A block to mRNA nuclear export in S. cerevisiae leads to hyperadenylation of transcripts that accumulate at the site of transcription. Mol Cell 7:887–898
Jensen TH, Dower K, Libri D, Rosbash M (2003) Early formation of mRNP: license for export or quality control. Mol Cell 11:1129–1138
Jensen TH, Boulay J, Olesen JR, Colin J, Weyler M, Libri D (2004) Modulation of transcription affects mRNP quality. Mol Cell 16:235–244
Johnson JM, Edwards S, Shoemaker D, Schadt EE (2005) Dark matter in the genome: evidence of widespread transcription detected by microarray tiling experiments. Trends Genet 21:93–102
Kammler S, Andersen SL, Jensen TH (2008) The RNA exosome component hRrp6 is a target for 5-fluorouracil in human cells. (in press)
Kaneko S, Rozenblatt-Rosen O, Meyerson M, Manley JL (2007) The multifunctional protein p54nrb/PSF recruits the exonuclease XRN2 to facilitate pre-mRNA 3′ processing and transcription termination. Genes Dev 21:1779–1789
Kim K, Klein R, Majewski J, Ott J (2004a) Estimating rates of alternative splicing in vertebrates and invertebrates. Nature Genet 36:915–917
Kim M, Krogan NJ, Vasiljeva L, Rando OJ, Nedea E, Greenblatt JF, Buratowski S (2004b) The yeast Rat1 exonuclease promotes transcription termination by RNA polymerase II. Nature 432:517–522
Kufel J, Allmang C, Petfalski E, Beggs J, Tollervey D (2003) Lsm Proteins are required for normal processing and stability of ribosomal RNAs. J Biol Chem 278:2147–2156
Kufel J, Bousquet-Antonelli C, Beggs JD, Tollervey D (2004) Nuclear pre-mRNA decapping and 5′ degradation in yeast require the Lsm2–8p complex. Mol Cell Biol 24:9646–9657
LaCava J, Houseley J, Saveanu C, Petfalski E, Thompson E, Jacquier A, Tollervey D (2005) RNA degradation by the exosome is promoted by a nuclear polyadenylation complex. Cell 121:713–724
Legrain P, Rosbash M (1989) Some cis- and trans-acting mutants for splicing target pre-mRNA to the cytoplasm. Cell 57:573–583
Lewis A, Felberbaum R, Hochstrasser M (2007) A nuclear envelope protein linking nuclear pore basket assembly, SUMO protease regulation, and mRNA surveillance. J Cell Biol 178:813–827
Libri D, Dower K, Boulay J, Thomsen R, Rosbash M, Jensen TH (2002) Interactions between mRNA export commitment, 3′-end quality control, and nuclear degradation. Mol Cell Biol 22:8254–8266
Lorentzen E, Conti E (2006) The exosome and the proteasome: nano-compartments for degradation. Cell 125:651–654
Lum PY, Armour CD, Stepaniants SB, Cavet G, Wolf MK, Butler JS, Hinshaw JC, Garnier P, Prestwich GD, Leonardson A, Garrett-Engele P, Rush CM, Bard M, Schimmack G, Phillips JW, Roberts CJ, Shoemaker DD (2004) Discovering modes of action for therapeutic compounds using a genome-wide screen of yeast heterozygotes. Cell 116:121–137
Lund MK, Guthrie C (2005) The DEAD-box protein Dbp5p is required to dissociate Mex67p from exported mRNPs at the nuclear rim. Mol Cell 20:645–651
Luo W, Johnson AW, Bentley DL (2006) The role of Rat1 in coupling mRNA 3′-end processing to transcription termination: implications for a unified allosteric-torpedo model. Genes Dev 20:954–965
Maniatis T, Reed R (2002) An extensive network of coupling among gene expression machines. Nature 416:499–506
Mattick JS (2003) Challenging the dogma: the hidden layer of non-protein-coding RNAs in complex organisms. Bioessays 25:930–939
Menon BB, Sarma NJ, Pasula S, Deminoff SJ, Willis KA, Barbara KE, Andrews B, Santangelo GM (2005) Reverse recruitment: the Nup84 nuclear pore subcomplex mediates Rap1/Gcr1/Gcr2 transcriptional activation. Proc Natl Acad Sci U S A 102:5749–5754
Milligan L, Torchet C, Allmang C, Shipman T, Tollervey D (2005) A nuclear surveillance pathway for mRNAs with defective polyadenylation. Mol Cell Biol 25:9996–10004
Minvielle-Sebastia L, Winsor B, Bonneaud N, Lacroute F (1991) Mutations in the yeast RNA14 and RNA15 genes result in an abnormal mRNA decay rate; sequence analysis reveals an RNA-binding domain in the RNA15 protein. Mol Cell Biol 11:3075–3087
Mitchell P, Petfalski E, Houalla R, Podtelejnikov A, Mann M, Tollervey D (2003) Rrp47p is an exosome-associated protein required for the 3′ processing of stable RNAs. Mol Cell Biol 23:6982–6992
Moore MJ (2002) Nuclear RNA turnover. Cell 108:431–434
Palancade B, Zuccolo M, Loeillet S, Nicolas A, Doye V (2005) Pml39, a novel protein of the nuclear periphery required for nuclear retention of improper messenger ribonucleoparticles. Mol Biol Cell 16:5258–5268
Petfalski E, Dandekar T, Henry Y, Tollervey D (1998) Processing of the precursors to small nucleolar RNAs and rRNAs requires common components. Mol Cell Biol 18:1181–1189
Proudfoot NJ (2003) Dawdling polymerases allow introns time to splice. Nat Struct Biol 10:876–878
Rigo F, Kazerouninia A, Nag A, Martinson HG (2005) The RNA tether from the poly(A) signal to the polymerase mediates coupling of transcription to cleavage and polyadenylation. Mol Cell 20:733–745
Rodriguez-Navarro S, Fischer T, Luo MJ, Antunez O, Brettschneider S, Lechner J, Perez-Ortin JE, Reed R, Hurt E (2004) Sus1, a functional component of the SAGA histone acetylase complex and the nuclear pore-associated mRNA export machinery. Cell 116:75–86
Roth KM, Wolf MK, Rossi M, Butler JS (2005) The nuclear exosome contributes to autogenous control of NAB2 mRNA levels. Mol Cell Biol 25:1577–1585
Rougemaille M, Gudipatif RK, Olesen JR, Thomsen R, Seraphin B, Libri D, Jensen TH (2007) Dissecting mechanisms of nuclear mRNA surveillance in THO/sub2 complex mutants. Embo J 26:2317–2326
Rutz B, Seraphin B (2000) A dual role for BBP/ScSF1 in nuclear pre-mRNA retention and splicing. Embo J 19:1873–1886
Saguez C, Schmid M, Olesen JR, Ghazy M, Qu X, Poulsen MB, Nasser T, Moore C, Jensen TH (2008) Nuclear mRNA surveillance in THO/sub2 mutants is triggered by inefficient polyadenylation. Mol Cell (in press)
Schmid M, Arib G, Laemmli C, Nishikawa J, Durussel T, Laemmli UK (2006) Nup-PI: the nucleopore-promoter interaction of genes in yeast. Mol Cell 21:379–391
Schroeder SC, Zorio DA, Schwer B, Shuman S, Bentley D (2004) A function of yeast mRNA cap methyltransferase, Abd1, in transcription by RNA polymerase II. Mol Cell 13:377–387
Schwer B, Mao X, Shuman S (1998) Accelerated mRNA decay in conditional mutants of yeast mRNA capping enzyme. Nucleic Acids Res 26:2050–2057
Sexton T, Schober H, Fraser P, Gasser SM (2007) Gene regulation through nuclear organization. Nature Struct Mol Biol 14:1049–1055
Stead JA, Costello JL, Livingstone MJ, Mitchell P (2007) The PMC2NT domain of the catalytic exosome subunit Rrp6p provides the interface for binding with its cofactor Rrp47p, a nucleic acid-binding protein. Nucleic Acids Res 35:5556–5567
Steinmetz EJ, Ng SB, Cloute JP, Brow DA (2006a) cis- and trans-Acting determinants of transcription termination by yeast RNA polymerase II. Mol Cell Biol 26:2688–2696
Steinmetz EJ, Warren CL, Kuehner JN, Panbehi B, Ansari AZ, Brow DA (2006b) Genome-wide distribution of yeast RNA polymerase II and its control by Sen1 helicase. Mol Cell 24:735–746
Strasser K, Masuda S, Mason P, Pfannstiel J, Oppizzi M, Rodriguez-Navarro S, Rondon AG, Aguilera A, Struhl K, Reed R, Hurt E (2002) TREX is a conserved complex coupling transcription with messenger RNA export. Nature 417:304–308
Taddei A, Van Houwe G, Hediger F, Kalck V, Cubizolles F, Schober H, Gasser SM (2006) Nuclear pore association confers optimal expression levels for an inducible yeast gene. Nature 441:774–778
Thiebaut M, Kisseleva-Romanova E, Rougemaille M, Boulay J, Libri D (2006) Transcription termination and nuclear degradation of cryptic unstable transcripts: a role for the nrd1-nab3 pathway in genome surveillance. Mol Cell 23:853–864
Thomsen R, Saguez C, Nasser T, Jensen TH (2008) General, rapid, and transcription-dependent fragmentation of nucleolar antigens in S. cerevisiae mRNA export mutants. RNA 4:706–716
Torchet C, Bousquet-Antonelli C, Milligan L, Thompson E, Kufel J, Tollervey D (2002) Processing of 3′-extended read-through transcripts by the exosome can generate functional mRNAs. Mol Cell 9:1285–1296
van Hoof A, Lennertz P, Parker R (2000) Yeast exosome mutants accumulate 3′-extended polyadenylated forms of U4 small nuclear RNA and small nucleolar RNAs. Mol Cell Biol 20:441–452
Vanacova S, Wolf J, Martin G, Blank D, Dettwiler S, Friedlein A, Langen H, Keith G, Keller W (2005) A new yeast poly(A) polymerase complex involved in RNA quality control. PLoS Biol 3:e189
Varani G (1997) A cap for all occasions. Structure 5:855–858
Vasiljeva L, Buratowski S (2006) Nrd1 interacts with the nuclear exosome for 3′ processing of RNA polymerase II transcripts. Mol Cell 21:239–248
Vinciguerra P, Iglesias N, Camblong J, Zenklusen D, Stutz F (2005) Perinuclear Mlp proteins downregulate gene expression in response to a defect in mRNA export. Embo J 24:813–823
Willingham AT, Gingeras TR (2006) TUF love for “junk” DNA. Cell 125:1215–1220
Wyers F, Rougemaille M, Badis G, Rousselle JC, Dufour ME, Boulay J, Regnault B, Devaux F, Namane A, Seraphin B, Libri D, Jacquier A (2005) Cryptic pol II transcripts are degraded by a nuclear quality control pathway involving a new poly(A) polymerase. Cell 121:725–737
Zenklusen D, Vinciguerra P, Wyss JC, Stutz F (2002) Stable mRNP formation and export require cotranscriptional recruitment of the mRNA export factors Yra1p and Sub2p by Hpr1p. Mol Cell Biol 22:8241–8253
Acknowledgments
We thank Pascal Preker and Francisco Malagon for critical reading of the manuscript, three anonymous referees for their valuable input, and Domenico Libri for sharing unpublished data. The work was supported by the Danish National Research Foundation (Grundforskningsfonden) and the Novo Nordisk Foundation. MS is the recipient of a Human Frontier Science Program long-term fellowship.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Communicated by E.A. Nigg
Rights and permissions
About this article
Cite this article
Schmid, M., Jensen, T.H. Quality control of mRNP in the nucleus. Chromosoma 117, 419–429 (2008). https://doi.org/10.1007/s00412-008-0166-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00412-008-0166-4