Abstract
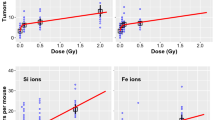
Gastrointestinal (GI) cancer risk among astronauts after encountering galactic cosmic radiation (GCR) is predicted to exceed safe permissible limits in long duration deep-space missions. Current predictions are based on relative biological effectiveness (RBE) values derived from in-vivo studies using single-ion beams, while GCR is essentially a mixed radiation field composed of protons (H), helium (He), and heavy ions. Therefore, a sequentially delivered proton (H) → Helium (He) → Oxygen (O) → Silicon (Si) beam was designed to simulate simplified-mixed-field GCR (Smf-GCR), and Apc1638N/+ mice were total-body irradiated to sham or γ (157Cs) or Smf-GCR followed by assessment of GI-tumorigenesis at 150 days post-exposure. Further, GI-tumor data from equivalent doses of heavy-ions (i.e., 0.05 Gy of O and Si) in 0.5 Gy of Smf-GCR were compared to understand the contributions of heavy-ions in GI-tumorigenesis. The Smf-GCR-induced tumor and carcinoma count were significantly greater than γ-rays, and male preponderance for GI-tumorigenesis was consistent with our earlier findings. Comparison of tumor data from Smf-GCR and equivalent doses of heavy ions revealed an association between higher GI-tumorigenesis where dose received from heavy-ions contributed to > 95% of the total GI-tumorigenic effect observed after Smf-GCR. This study provides the first experimental evidence that cancer risk after GCR exposure could largely depend on doses received from constituent heavy-ions.


Similar content being viewed by others
References
Barcellos-Hoff MH et al (2015) Concepts and challenges in cancer risk prediction for the space radiation environment. Life Sci Space Res (Amst) 6:92–103
Chancellor JC et al (2018) Limitations in predicting the space radiation health risk for exploration astronauts. NPJ Microgravity 4:8
Cheema AK et al (2014) Long-term differential changes in mouse intestinal metabolomics after γ and heavy ion radiation exposure. PLoS One 9:e87079
Cucinotta FA et al (2020) Benchmarking risk predictions and uncertainties in the NSCR model of GCR cancer risks with revised low let risk coefficients. Life Sci Space Res (Amst) 27:64–73
Cucinotta FA et al (2021) A proposed change to astronaut exposures limits is a giant leap backwards for radiation protection. Life Sci Space Res (Amst) 31:59–70
Curtis SB (1996) Possible effects of protracted exposure on the additivity of risks from space radiations. Adv Space Res 18:41–44
Datta K et al (2012) Exposure to heavy ion radiation induces persistent oxidative stress in mouse intestine. PLoS One 7:e42224
Ehresmann B et al (2016) Charged particle spectra measured during the transit to mars with the mars science laboratory radiation assessment detector (MSL/RAD). Life Sci Space Res (Amst) 10:29–37
Fokas E et al (2009) Ion beam radiobiology and cancer: time to update ourselves. Biochim Biophys Acta 1796:216–229
Huang EG et al (2020) Simulating galactic cosmic ray effects: synergy modeling of murine tumor prevalence after exposure to two one-ion beams in rapid sequence. Life Sci Space Res (Amst) 25:107–118
Kiffer FC et al (2022) Effects of a 33-ion sequential beam galactic cosmic ray analog on male mouse behavior and evaluation of CDDO-EA as a radiation countermeasure. Behav Brain Res 419:113677
Kim E et al (2018) Cell signaling heterogeneity is modulated by both cell-intrinsic and -extrinsic mechanisms: an integrated approach to understanding targeted therapy. PLoS Biol 16:e2002930
Kumar S et al (2018) Space radiation triggers persistent stress response, increases senescent signaling, and decreases cell migration in mouse intestine. Proc Natl Acad Sci USA 115:E9832–E9841
Kuraguchi M et al (2001) The distinct spectra of tumor-associated Apc mutations in mismatch repair-deficient Apc1638N mice define the roles of MSH3 and MSH6 in DNA repair and intestinal tumorigenesis. Cancer Res 61:7934–7942
Lacina L et al (2015) Cancer microenvironment: what can we learn from the stem cell niche. Int J Mol Sci 16:24094–24110
Matsumoto Y et al (2015) Dependence of the bystander effect for micronucleus formation on dose of heavy-ion radiation in normal human fibroblasts. Radiat Prot Dosimetry 166:152–156
Mehner C et al (2021) Real versus simulated galactic cosmic radiation for investigating cancer risk in the hematopoietic system - are we comparing apples to apples. Life Sci Space Res (Amst) 29:8–14
Mohan R et al (2018) Proceedings of the national cancer institute workshop on charged particle radiobiology. Int J Radiat Oncol Biol Phys 100:816–831
Norbury JW et al (2016) Galactic cosmic ray simulation at the NASA space radiation laboratory. Life Sci Space Res (Amst) 8:38–51
Shuryak I et al (2017) Scaling human cancer risks from low LET to high LET when dose-effect relationships are complex. Radiat Res 187:476–482
Simonsen LC et al (2020) NASA’s first ground-based galactic cosmic ray simulator: enabling a new era in space radiobiology research. PLoS Biol 18:e3000669
Smits R et al (1998) Apc1638N: a mouse model for familial adenomatous polyposis-associated desmoid tumors and cutaneous cysts. Gastroenterology 114:275–283
Sont WN et al (2001) First analysis of cancer incidence and occupational radiation exposure based on the national dose registry of Canada. Am J Epidemiol 153:309–318
Strigari L et al (2021) Dose-effects models for space radiobiology: an overview on dose-effect relationships. Front Public Health 9:733337
Suman S et al (2016) Relative biological effectiveness of energetic heavy ions for intestinal tumorigenesis shows male preponderance and radiation type and energy dependence in APC(1638N/+) mice. Int J Radiat Oncol Biol Phys 95:131–138
Suman S et al (2017) Low and high dose rate heavy ion radiation-induced intestinal and colonic tumorigenesis in APC1638N/+ mice. Life Sci Space Res (Amst) 13:45–50
Suman S et al (2020) Heavy ion space radiation triggers ongoing DNA base damage by downregulating DNA repair pathways. Life Sci Space Res (Amst) 27:27–32
Suman S et al (2021) Effects of dietary aspirin on high-LET radiation-induced prostaglandin E2 levels and gastrointestinal tumorigenesis in Apc1638N/+ mice. Life Sci Space Res (Amst) 31:85–91
Tomita M et al (2015) Nitric oxide-mediated bystander signal transduction induced by heavy-ion microbeam irradiation. Life Sci Space Res (Amst) 6:36–43
Trani D et al (2013) Sex-dependent differences in intestinal tumorigenesis induced in Apc1638N/+ mice by exposure to γ rays. Int J Radiat Oncol Biol Phys 85:223–229
Vicente-Dueñas C et al (2018) Epigenetic priming in cancer initiation. Trends Cancer 4:408–417
Wada M et al (2013) Modeling the biological response of normal human cells, including repair processes, to fractionated carbon beam irradiation. J Radiat Res 54:798–807
Wu S et al (2018) Evaluating intrinsic and non-intrinsic cancer risk factors. Nat Commun 9:3490
Yahyapour R et al (2018) Radiation-induced non-targeted effect and carcinogenesis; implications in clinical radiotherapy. J Biomed Phys Eng 8:435–446
Zeitlin C et al (2013) Measurements of energetic particle radiation in transit to mars on the mars science laboratory. Science 340:1080–1084
Acknowledgements
We are thankful to the National Aeronautics and Space Administration (NASA) for funding this study through grant# NNX09AU95G and 80NSSC22K1279. We highly appreciate the outstanding support provided by Dr. Adam Rusek, Dr. Peter Guida, and all the staff members of the NASA Space Radiation Laboratory (NSRL) to conduct space radiation exposures. We also acknowledge Ms. Ake Pelagie for animal colony maintenance.
Author information
Authors and Affiliations
Contributions
SS, KD, and AF designed the study; SS, BK, KD, SK, BM, and JA performed the experiments; SS wrote the manuscript and incorporated inputs from all the co-authors.
Corresponding author
Ethics declarations
Conflict of interest
All listed authors declare that they do not have any competing interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Suman, S., Kumar, S., Kallakury, B.V.S. et al. Predominant contribution of the dose received from constituent heavy-ions in the induction of gastrointestinal tumorigenesis after simulated space radiation exposure. Radiat Environ Biophys 61, 631–637 (2022). https://doi.org/10.1007/s00411-022-00997-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00411-022-00997-z