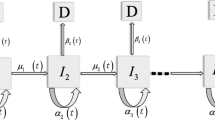
Abstract
Lung cancer mortality after radon exposure in the Wismut cohort was analyzed using the two-stage clonal expansion (TSCE) model. A total of 2996 lung cancer deaths among the 58,695 male workers were observed during the follow-up period between 1946 and 2003. Adjustment to silica exposure was performed to find a more accurate estimation of the risk of radon exposure. An additional analysis with the descriptive excess relative risk (ERR) model was carried out for comparison. The TSCE model that best describes the data is nonlinear in the clonal expansion with radon exposure and has a saturation level at an exposure rate of \(d_{\mathrm{r}}\simeq 100\,\text{WLM}{/}\text{yr}\). The excess relative risk decreases with age and shows an inverse exposure rate effect. In comparison with the ERR model, the TSCE model predicts a considerably larger risk for low exposures rates below \(50\,\text{WLM}{/}\text{yr}\). Comparison to other mechanistic studies of lung cancer after exposure to alpha particles using the TSCE model reveals an extraordinary consistency in the main features of the exposure response, given the diversity in the characteristics of the cohorts and the exposure across different studies. This suggests that a nonlinear response mechanism in the clonal expansion, with some level of saturation at large exposure rates, may be playing a crucial role in the development of lung cancer after alpha particle irradiation.





Similar content being viewed by others
Notes
One working level, 1 WL, is \(1.3\times 10^5\) MeV of \(\alpha\)-particle energy per liter of air. One WLM of exposure corresponds to one WL during 1 working month or 170 h.
Since the TSCE model is formulated with rate parameters, the silica rate and not the total silica exposure is used as input, similar to the radon exposure rate. These rates are integrated over lifetime to obtain the total risk. It is nevertheless interesting to note that the weekly silica exposure obtained here is of the same order of magnitude as the NIOSH recommended limit for respirable silica of \(0.05\,\mathrm{mg}{/}\mathrm{m}^3\) (NIOSH 1974).
One is inclined to assume that extrapolating the risk with age in a model based on a dynamical process is more reliable than in a purely descriptive model (BEIR 1999). The TSCE model has more flexibility to accommodate different functional forms for the hazard and survival function at different attained ages (Moolgavkar and Venzon 1979; Moolgavkar and Knudson 1981), and may therefore provide more accurate fits to the data on the intermediate and young ages regime.
These models were referred to as exposure-age-concentration and exposure-age-duration models.
References
Akaike H (1973) Information theory and extension of the maximum likelihood principle. In: Proceedings of the second international symposium on information theory. Akademia Kiado, Budapest
Balashazy I, Farkas A, Madas BG et al (2009) Non-linear relationship of cell hit and transformation probabilities in a low dose of inhaled radon progenies. J Radiol Prot 29:147–162
Barrett J (1993) Mechanisms of multistep carcinogenesis and carcinogen risk assessment. Environ Health Perspect 100:9–20
BEIR VI (1999) Health effects of exposure to radon. Committee on the biological effects of ionizing radiation, National Research Council. National Academy Press, Washington, DC
Borm P, Tran L, Donaldson K (2011) The carcinogenic action of crystalline silica: a review of the evidence supporting secondary inflammation-driven genotoxicity as a principal mechanism. Crit Rev Toxicol 41(9):756–770
Brenner DJ, Hall EJ (1990) The inverse dose rate effect for oncogenic transformation by neutrons and charged particles. a plausible interpretation consistent with published data. Radiat Biol 58:745–758
Brenner DJ, Hall EJ, Randers-Pehrson G et al (1993) Mechanistic considerations on the dose-rate/let dependence of oncogenic transformation by ionizing irrariation. Radiat Res 133:365–369
Brugmans M, Rispens S, Bijwaard H (2004) Radon-induced lung cancer in French and Czech miner cohorts described with a two-mutation cancer model. Radiat Environ Biophys 43:153–163
Cross F (1992) A review of experimental animal radon health effects data. In: Radiation research: vol 11: a twentieth-century perspective. Academic Press, San Diego, pp 476–481
Dahmann D, Bauer H, Stoyke G et al (2008) Retrospective exposure assesment for respirable and inhalable dust, crystalline silica and arsenic in the former German uranium mines of SAG/SDAG Wismut. Int Arch Occup Environ Health 81:949–958
Diet, Nutrition, and Cancer. National Research Council (US) (1982) Committee on diet, nutrition, and cancer. National Academies Press, Washington
Dobson A, Barnett A (2008) An introduction to generalized linear models, 3rd edn. Chapman & Hall, Boca Raton texts in statistical science series
Eidemüller M, Ostroumova E, Krestinina L, Epiphanova S, Akleyev A, Jacob P (2010) Comparison of mortality and incidence solid cancer risk after radiation exposure in the Techa River Cohort. Radiat Environ Biophys 49:477–490
Eidemüller M, Jacob P, Lane R et al (2012) Lung cancer mortality (1950–1999) among Eldorado uranium workers: a comparison of models of carcinogenesis and empirical excess risk models. PloS ONE 7(8):e41431
Felter S, Conolly R, Bercu J et al (2011) A proposed framework for assessing risk from less-than-lifetime exposures to carcinogens. Crit Rev Toxicol 41(6):507–544
Grosche B, Kreuzer M, Kreisheimer M et al (2006) Lung cancer risk among German male uranium miners: a cohort study, 1946–1998. Br J Cancer 95:1280–1287
Hanahan D, Weinberg R (2000) The hallmarks of cancer. Cell 100:57–70
Hanahan D, Weinberg R (2011) The hallmarks of cancer: the next generation. Cell 144:646–674
Hazelton W, Luebeck E, Heidenreich F, Moolgavkar S (2001) Analysis of a historical cohort of Chinese tin miners with arsenic, cigarette smoke, and pipe smoke exposures using the biologically based two-stage clonal expansion model. Radiat Res 156:78–94
Heidenreich W (1996) On the parameters of the clonal expansion model. Radiat Environ Biophys 35:127–129
Heidenreich W, Luebeck E, Moolgavkar S (1997) Some properties of the hazard function of the two-mutation clonal expansion model. Risk Anal 17:391–399
Heidenreich W, Jacob P, Paretzke HG et al (1999) Two-step model for the risk of fatal and incidental lung tumors in rats exposed to radon. Radiat Res 151:209–217
Heidenreich W, Tomasek L, Rogel A (2004) Studies of radon-exposed miner cohorts using a biological based model: comparison of current Czech abd French data with historic data from China and Colorado. Radiat Environ Biophys 43:247–256
Heidenreich W, Tomasek L, Grosche B et al (2012) Lung cancer mortality in the European uranium miners cohort analysed with a biologically based model taking into account radon measurement error. Radiat Environ Biophys 51:263–275
IARC (1997) Silica, some silicates, coal dust and paraaramide fibrils, IARC monographs on the evaluation og the carcinogenic risk of chemicals to humans, vol 68. International Agency for Research, Lyon in Cancer
ICRP 2010 (2010) Lung cancer risk from radon and progeny and statement on radon. ICRP Publication 115, ann. ICRP 40(1)
Iyer R, Lehnert BE (2000) Factors underlying the cell growth-related bystander responses to \(\alpha\) particles. Cancer Res 60:1290–1298
Jacob V, Jacob P, Meckbach R et al (2005) Lung cancer in Mayak workers: interaction of smoking and plutonium exposure. Radiat Environ Biophys 44:119–129
Jacob P, Meckbach R, Sokolnikov M et al (2007) Lung cancer risk of Mayak workers: modelling of carcinogenesis and bystander effect. Radiat Environ Biophys 46:383–394
James F (1994) Minuit function minimization and error analysis version 94.1. Technical report, CERN, Geneva
Kai M, Luebeck E, Moolgavkar S (1997) Analysis of the incidence of solid cancer among atomic bomb survivors using a two-stage model of carcinogenesis. Radiat Res 148:348–358
Kaiser J, Jacob P, Meckbach R, Cullings H (2012) Breast cancer risk in atomic bomb survivors from multi-model inference with incidence data 1958–1998. Radiat Environ Biophys 51:1–14
Kaiser J, Meckbach R, Jacob P (2014) Genomic instability and radiation risk in molecular pathways to colon cancer. PloS ONE 9(10):e111024
Kreuzer M, Schnelzer M, Tschense A et al (2010a) Cohort profile: the German uranium miners cohort study. Int J Epidemiol 39:980–987
Kreuzer M, Walsh L, Schnelzer M et al (2010b) Radon and risk of death from cancer and cardiovascular diseases in the German uranium miners cohort study. Radiat Environ Biophys 49:177–185
Kreuzer M, Fenske N, Schnelzer M et al (2015) Lung cancer risk at low radon exposures rates in German uranium miners. Br J Cancer 113:1367–1369
Lane R, Frost S, Howe G, Zablotska L (2010) Mortality (1950–1999) and cancer incidence (1969–1999) in the cohort of Eldorado uranium workers. Radiat Res 174:773–785
Leenhouts E (1999) Radon-induced lung cancer in smokers and non-smokers: risk implications using a two-mutation carcinogenesis model. Radiat Environ Biophys 38:57–71
Lehmann F, Hambeck L, Linkert K et al (1998) Belastung durch ionisierende Strahlung im Uranerzbergbau der ehemaligen DDR. Hauptverband der gewerblichen Berufsgenossenschaften, St. Augustim
Leng S, Thomas CL, Snider AM et al (2010) Macrophage diversity enhances tumor progression and metastasis. Cell 141:39–41
Leuraud K, Schnelzer M, Tomasek L et al (2011) Radon, smoking and lung cancer risk: results of a joint analysis of three European case-control studies among uranium miners. Radiat Res 176:375–387
Little M, Li G (2007) Stochastic modelling of colon cancer: is there a role for genomic instability? Carcinogenesis 28:479–487
Little M, Haylock R, Muirhead C (2002) Modelling lung tumor risk in radon-exposed uranium miners using generalizations of the two mutation model of Venzon and Knudson. Int J Radiat Biol 78:49–68
Lubin L, Boice J, Edling C et al (1994) Radon and Lung cancer risk: a joint analysis of 11 underground miners studies, NIH publication No. 94-3644, National institutes of Health, Bethesda, MD
Lubin J, Boice J, Edling C et al (1995) Radon-exposed underground miners and inverse dose-rate (protraction enhancements) effects. Health Phys 69:494–500
Luebeck E, Heidenreich W, Hazelton W et al (1999a) Biologically based analysis of the data for the Colorado uranium miners cohort: age, dose and dose-rate effects. Radiat Res 152:339–351
Luebeck EG, Curtis SB, Cross FT et al (1999b) Two-stage model of radon induced malignant lung tumors in rats. Radiat Res 145:163–173
Luebeck EG, Curtius K, Jeon J, Hazelton WD (2013) Impact of tumor progression on cancer incidence curves. Cancer Res 73:1086–1096
Madas BG, Balashazy I (2011) Mutation induction by inhaled radon progeny modeled at the tissue level. Radiat Environ Biophys 50:553–570
Madas BG, Vargas K (2014) Biophysical modelling of the effects of inhaled radon progeny on the bronchial epithelium for the estimation of the relationships applied in the two-stage clonal expansion model of carcinogenesis. Radiat Prot Dosimetry 50:237–241
Mantovani A, Allavena P, Sica A, Balkwill F (2008) Cancer-related inflammation. Nature 454:436–444
Moolgavkar S (1983) Model of human carcinogenesis: action of environmental agents. Environ Health Perspect 50:285–291
Moolgavkar S, Venzon D (1979) Two event models for carcinogenesis: incidence curves for childhood and adult tumors. Math Biosci 47:55–77
Moolgavkar S, Knudson A (1981) Mutation and cancer: a model for human carcinogenesis. J Natl Cancer Inst 66:1037–1052
Moolgavkar S, Luebeck E (1990) Two-event model for carcinogenesis: biological, mathematical and statistical consideration. Risk Anal 10:323–341
Network The Cancer Genome Atlas Research (2012) Comprehensive genomic characterization of squamous cell lung cancers. Nature 519:525
Network The Cancer Genome Atlas Research (2014) Comprehensive molecular profiling of lung adenocarcinoma. Nature 511:543–550
Neumann H (2009) Risk assesment of chemical carcinogens and thresholds. Crit Rev Toxicol 39(6):449–461
NIOSH (1974) National Institute for Occupational Safety and Health (NIOSH). Criteria for a recommended standard: occupational exposure to crystalline silica. DHEW (NIOSH) Publication No. 75–120
Portier H, Edler L (2009) Two-stage models of carcinogenesis, classification of agents, and design experiments. Fundam Appl Toxicol 14:444–460
Qian B, Pollard J (2010) Macrophage diversity enhances tumor progression and metastasis. Cell 141:39–41
Raue A, Kreuz C, Maiwald T, Bachmann J, Schilling M, Klingmüller U, Timmer J (2009) Structural and practical identifiability analysis of partially observed dynamical models by exploiting the profile likelihood. Bioinformatics 25(209):1923–1929
Raue A, Becker V, Klingmüller U, Timmer J (2010) Identifiability and observability analysis for experimental design in nonlinear dynamical models. Bioinformatics 25(209):1923–1929
Reifen R (2002) Vitamin a as an anti-inflammatory agent. Proc Nutr Soc 61:397–400
Rogel A, Laurier D, Tirmarche M et al (2002) Lung cancer risk in the French cohort of uranium miners. J Radiol Prot 22:A101–A106
Rossi HH, Kellerer AM (1986) The dose rate dependence of oncogenic transformation by neutrons may be due to variation of response during the cell cycle. Int J Radiat Biol 50:353–361
Schnelzer M, Hammer G, Kreuzer M et al (2010) Accounting for smoking in the radon-related lung cancer risk among German uranium miners: results of a nested case–control study. Health Phys 98:20–28
Schoenberg B (1983) Calculating confidence intervals for rates and ratios. Neuroepidemiology 2:257–265
Schubauer-Berigan M, Daniels R, P LE (2009) Radon exposure and mortality among white and American Indian uranium miners: an update of the Colorado plateau cohort. Am J Epidemiol 174:718–730
Sogl M, Taeger D, Pallapies D et al (2012) Quantitative relationship between silica exposure and lung cancer mortality in German uranium miners, 1946–2003. Br J Cancer 107:1188–1194
Sprung CN, Ivashkevich A, Forrester HB et al (2015) Oxidative DNA damage caused by inflammation may link to stress-induced non-targeted effects. Cancer Lett 356:72–81
Tan W (1991) Chapter 2: two-stage models of carcinogenesis, stochastic models of carcinogenesis. Marcel Dekker, Inc, New York
Tomasek L (1999) Czech miner studies of lung cancer risk from radon. J Radiol Prot 22:A107–A112
Tomasek L, Placek V (1999) Radon exposure and lung cancer risk: Czech cohort study. Radiat Res 152:339–351
Tomasek L, Rogel A, Tirmarche M, Mitton N, Laurier D (2008) Lung cancer in French and Czech uranium miners: radon-associated risk at low exposure rates and modifying effects of time since exposure and age at exposure. Radiat Res 169:125–137
Tomasetti C, Vogelstein B (2015) Variation in cancer risk among tissues can be explained by the number of stem cell divisions. Science 347:78–81
UNSCEAR (2000) Sources and effects of ionizing radiation, vol II. Effects. United Nations, New York, p 2000
Vacquier B, Caer S, Rogel A et al (2008) Mortality risk in the French cohort of uranium miners: extended follow-up 1946–1999. Occup Environ Med 65:597–604
van Dillen T, Dekkers F, Bijwaard H et al (2011) Lung cancer from radon: a two-stage model analysis of the WISMUT cohort, 1955–1998. Radiat Res 175:119–130
Vogelstein B, Papadopoulos N, Velculescu V, Zhou S, Diaz L, Kinzler K (2013) Cancer genome landscapes. Science 339:1546–1558
Walsh L (2007) A short review of model selection techniques for radiation epidemiology. Radiat Environ Biophys 46:205–213
Walsh L, Dufey F, Tschense A et al (2010) Radon and the risk of cancer mortality—internal Poisson models for the German uranium miners cohort. Health Phys 99:292–300
WHO (2007) Harmonization project document no. 4: part I: IPCS framework for analysing the relevance of cancer mode of action for humans and case studies
Winkler-Heil R, Hofman W (2007) Comparison radon lung dosimetry models for the estimation of dose uncertainties. Radiat Prot Dosimetry 127:27–30
Wood CE, Hukkanen RR, Sura R et al (2015) The scientific and regulatory policy committee review: interpretation and use of cell proliferation data in cancer risk assesment. Toxicol Pathol 43:760–775
Zöllner S, Sokolnikov M, Eidemüller M (2015) Beyond two-stage models for lung carcinogenesis in the Mayak workers. Mutat Res 775:1–9
Acknowledgments
We thank the Federal Office for Radiation Protection (BfS) for providing the data on the Wismut cohort. It is a pleasure to thank Michaela Kreuzer, Linda Walsh and Florian Dufey for useful discussions about the cohort, and Jan Christian Kaiser, Reinhard Meckbach and Sascha Zöllner for helpful comments about the models. This work was supported by the European Commission under FP7 project EpiRadBio with Project No. 269553.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zaballa, I., Eidemüller, M. Mechanistic study on lung cancer mortality after radon exposure in the Wismut cohort supports important role of clonal expansion in lung carcinogenesis. Radiat Environ Biophys 55, 299–315 (2016). https://doi.org/10.1007/s00411-016-0659-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00411-016-0659-0