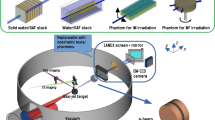
Abstract
The long-term goal to integrate laser-based particle accelerators into radiotherapy clinics not only requires technological development of high-intensity lasers and new techniques for beam detection and dose delivery, but also characterization of the biological consequences of this new particle beam quality, i.e. ultra-short, ultra-intense pulses. In the present work, we describe successful in vivo experiments with laser-driven electron pulses by utilization of a small tumour model on the mouse ear for the human squamous cell carcinoma model FaDu. The already established in vitro irradiation technology at the laser system JETI was further enhanced for 3D tumour irradiation in vivo in terms of beam transport, beam monitoring, dose delivery and dosimetry in order to precisely apply a prescribed dose to each tumour in full-scale radiobiological experiments. Tumour growth delay was determined after irradiation with doses of 3 and 6 Gy by laser-accelerated electrons. Reference irradiation was performed with continuous electron beams at a clinical linear accelerator in order to both validate the dedicated dosimetry employed for laser-accelerated JETI electrons and above all review the biological results. No significant difference in radiation-induced tumour growth delay was revealed for the two investigated electron beams. These data provide evidence that the ultra-high dose rate generated by laser acceleration does not impact the biological effectiveness of the particles.





Similar content being viewed by others
References
Acharya S, Bhat NN, Joseph P, Sanjeev G, Sreedevi B, Narayana Y (2011) Dose rate effect on micronuclei induction in human blood lymphocytes exposed to single pulse and multiple pulses of electrons. Radiat Environ Biophys 50:253–263. doi:10.1007/s00411-011-0353-1
Baumann M, Bentzen SM, Doerr W, Joiner MC, Saunders M, Tannock IF, Thames HD (2001) The translational research chain: is it delivering the goods? Int J Radiat Oncol Biol Phys 49:345–351. doi:10.1016/S0360-3016(00)01483-8
Beyreuther E et al (2010) Establishment of technical prerequisites for cell irradiation experiments with laser-accelerated electrons. Med Phys 37:1392–1400. doi:10.1118/1.3301598
Bin JH et al (2012) A laser-driven nanosecond proton source for radiobiological studies. Appl Phys Lett 101:243701. doi:10.1063/1.4769372 (4 pp)
Brüchner K, Beyreuther E, Baumann M, Krause M, Oppelt M, Pawelke J (2014) Establishment of a small animal tumour model for in vivo studies with low energy laser accelerated particles. Radiat Oncol 9:57. doi:10.1186/1748-717X-9-57 (9 pp)
Chiu C, Fomytskyi M, Grigsby F, Raischel F, Downer MC, Tajima T (2004) Laser electron accelerators for radiation medicine: a feasibility study. Med Phys 31:2042–2052. doi:10.1118/1.1739301
Daido H, Nishiuchi M, Pirozhkov AS (2012) Review of laser-driven ion sources and their applications. Rep Prog Phys 75:056401. doi:10.1088/0034-4885/75/5/056401 (71 pp)
Doria D et al (2012) Biological effectiveness on live cells of laser driven protons at dose rates exceeding 109 Gy/s. AIP Adv 2:011209. doi:10.1063/1.3699063 (6 pp)
Favaudon V et al (2014) Ultrahigh dose-rate FLASH irradiation increases the differential response between normal and tumor tissue in mice. Sci Transl Med 6:245ra93. doi:10.1126/scitranslmed.3008973 (9 pp)
Folkman J (2002) Role of angiogenesis in tumour growth and metastasis. Semin Oncol 29(6 Suppl 16):15–18. doi:10.1053/sonc.2002.37263
Fuchs T, Szymanowski H, Oelfke U, Glinec Y, Rechatin C, Faure J, Malka V (2009) Treatment planning for laser-accelerated very-high energy electrons. Phys Med Biol 54:3315–3328. doi:10.1088/0031-9155/54/11/003
Glinec Y, Faure J, Malka V, Fuchs T, Szymanowski H, Oelfke U (2006) Radiotherapy with laser-plasma accelerators: Monte Carlo simulation of dose deposited by an experimental quasimonoenergetic electron beam. Med Phys 33:155–162. doi:10.1118/1.2140115
Greubel C et al (2011) Scanning irradiation device for mice in vivo with pulsed and continuous proton beams. Radiat Environ Biophys 50:339–344. doi:10.1007/s00411-011-0365-x
Hall EJ (1972) Radiation dose-rate: a factor of importance in radiobiology and radiotherapy. Br J Radiol 45:81–97. doi:10.1259/0007-1285-45-530-81
Hunter N, Muirhead CR (2009) Review of relative biological effectiveness dependence on linear energy transfer for low-LET radiations. J Radiol Prot 29:5–21. doi:10.1088/0952-4746/29/1/R01
IAEA (2000) Absorbed dose determination in external beam radiotherapy: an international code of practice for dosimetry based on standards of absorbed dose on water. Technical reports series 398. International Atomic Energy Agency, Vienna, p 229
Karsch L, Beyreuther E, Burris-Mog T, Kraft S, Richter C, Zeil K, Pawelke J (2012) Dose rate dependence for different dosimeters and detectors: TLD, OSL, EBT films, and diamond detectors. Med Phys 39:2447–2455. doi:10.1118/1.3700400
Kraft SD et al (2010) Dose-dependent biological damage of tumour cells by laser-accelerated proton beams. New J Phys 12:085003. doi:10.1088/1367-2630/12/8/085003 (12 pp)
Krause A, Hessel F, Zips D, Hilberg F, Baumann M (2004) Adjuvant inhibition of the epidermal growth factor receptor after fractionated irradiation of FaDu human squamous cell carcinoma. Radiother Oncol 72:95–101. doi:10.1016/j.radonc.2004.05.003
Laschinsky L et al (2012) Radiobiological effectiveness of laser accelerated electrons in comparison to electron beams from a conventional linear accelerator. J Radiat Res 53:395–403. doi:10.1269/Jrr.11080
Ledingham KWD, Galster W (2010) Laser-driven particle and photon beams and some applications. New J Phys 12:045005. doi:10.1088/1367-2630/12/4/045005 (66 pp)
Ledingham KWD, Galster W, Sauerbrey R (2007) Laser-driven proton oncology—a unique new cancer therapy? Br J Radiol 80:855–858. doi:10.1259/Bjr/29504942
Leemans WP et al (2006) GeV electron beams from a centimetre-scale accelerator. Nat Phys 2:696–699. doi:10.1038/Nphys418
Linz U, Alonso J (2007) What will it take for laser driven proton accelerators to be applied to tumor therapy? Phys Rev Spec Top Accel Beams 10:094801. doi:10.1103/Physrevstab.10.094801 (8 pp)
Lundh O et al (2011) Few femtosecond, few kiloampere electron bunch produced by a laser-plasma accelerator. Nat Phys 7:219–222. doi:10.1038/Nphys1872
Malka V, Faure J, Gauduel YA (2010) Ultra-short electron beams based spatio-temporal radiation biology and radiotherapy. Mutat Res 704:142–151. doi:10.1016/j.mrrev.2010.01.006
Nicolai M et al (2014) Realizing a laser-driven electron source applicable for radiobiological tumour irradiation. Appl Phys B 116:643–651. doi:10.1007/s00340-013-5747-0
Rangan SRS (1972) New human cell line (Fadu) from a hypopharyngeal carcinoma. Cancer 29:117–121. doi:10.1002/1097-0142(197201)29:1<117:Aid-Cncr2820290119>3.0.Co;2-R
Richter C, Pawelke J, Karsch L, Woithe J (2009) Energy dependence of EBT-1 radiochromic film response for photon (10 kVp–15 MVp) and electron beams (6–18 MeV) readout by a flatbed scanner. Med Phys 36:5506–5514. doi:10.1118/1.3253902
Richter C et al (2011) Dosimetry of laser-accelerated electron beams used for in vitro cell irradiation experiments. Radiat Meas 46:2006–2009. doi:10.1016/j.radmeas.2011.04.019
Schardt D, Elsasser T, Schulz-Ertner D (2010) Heavy-ion tumour therapy: physical and radiobiological benefits. Rev Mod Phys 82:383–425. doi:10.1103/RevModPhys.82.383
Schnell M et al (2012) Deducing the electron-beam diameter in a laser-plasma accelerator using X-ray betatron radiation. Phys Rev Lett 108:075001. doi:10.1103/Physrevlett.108.075001 (5 pp)
Schürer M et al (2012) Irradiation system for pre-clinical studies with laser accelerated electrons. Biomed Tech 57(Suppl. 1):62–65. doi:10.1515/bmt-2012-4244
Sen A et al (2011) Mild elevation of body temperature reduces tumour interstitial fluid pressure and hypoxia and enhances efficacy of radiotherapy in murine tumour models. Cancer Res 71:3872–3880. doi:10.1158/0008-5472.CAN-10-4482
Suit HD, Shalek RJ, Wette R (1965) Radiation response of C3H mouse mammary carcinoma evaluated in terms of cellular radiation sensitivity. In: Cellular Radiation Biology. Williams and Wilkins, Baltimore, pp 514–530
Yogo A et al (2011) Measurement of relative biological effectiveness of protons in human cancer cells using a laser-driven quasimonoenergetic proton beamline. Appl Phys Lett 98:053701. doi:10.1063/1.3551623 (3 pp)
Zeil K et al (2013) Dose-controlled irradiation of cancer cells with laser-accelerated proton pulses. Appl Phys B 110:437–444. doi:10.1007/s00340-012-5275-3
Zlobinskaya O et al (2014) The effects of ultra-high dose rate proton irradiation on growth delay in the treatment of human tumour xenografts in nude mice. Radiat Res 181(2):177–183. doi:10.1667/RR13464.1
Acknowledgments
The authors are grateful to Mrs. D. Pfitzmann and Ms. K. Schumann for their excellent technical assistance and Mr. F. Tillner for performing the dosimetry at the 200 kV X-ray tube in Dresden. Thanks to the laser engineers from the JETI crew and to the medical physicists at LINAC for their support and ongoing interest in our studies. This work was supported by the Bundesministerium für Bildung und Forschung (BMBF, Grant Numbers 03ZIK445, 03Z1N511, 03Z1H531 and 05K10SJ2) and by the DFG (TR18).
Conflict of interest
The authors declare that they have no conflict of interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Oppelt, M., Baumann, M., Bergmann, R. et al. Comparison study of in vivo dose response to laser-driven versus conventional electron beam. Radiat Environ Biophys 54, 155–166 (2015). https://doi.org/10.1007/s00411-014-0582-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00411-014-0582-1