Abstract.
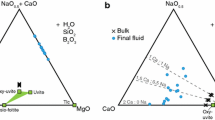
Tourmaline has been synthesized hydrothermally at 200 MPa between 300 and 700 °C from oxide mixtures with Mg–Al ratios for the end members dravite NaMg3Al6(Si6O18)(BO3)3(OH)3(OH) and Mg-foitite ❏(Mg2Al)Al6 (Si6O18)(BO3)3(OH)3(OH). Six different Na concentrations were investigated to determine the distribution of Na between tourmaline and fluid in the SiO2-saturated system Na2O–MgO–Al2O3–SiO2–B2O3–H2O–HCl. Synthetic tourmaline ranges from X-site vacant (❏) tourmaline (Mg-foitite) to nearly ideal dravite with Na=0.95 apfu. There are small, but significant, amounts of proton deficiency and negligible tetrahedral Al. Chemical variation is primarily caused by the substitutions Al❏Mg–1Na–1 and minor AlMg–1H–1. Varying amounts of Na and ❏ determine the Mg/Al ratios. Besides tourmaline and quartz, additional Mg–Al phases are chlorite and, at 700 °C, cordierite. Albite is also present at high Na concentrations in the bulk composition. The c dimension of the tourmaline crystals increases with Na in tourmaline. The amount of Na in the X-site depends strongly on the bulk concentration of Na in the system as well as on the temperature. These factors in turn control the phase assemblage and the composition of the fluid phase. For the assemblage tourmaline + quartz + chlorite/cordierite + fluid, a linear relationship exists between Na concentration in the fluid (quenched after the run) and tourmaline with temperature: T °C [±25 °C]=(Na<SUB>fluid</SUB>/Na<SUB>tur</SUB>)×558.878-14.692 (<I>r</I><SUP>2</SUP>=0.96).
For the assemblage tourmaline + albite + quartz + fluid, it is: T °C [±15 °C]=(Na<SUB>fluid</SUB>/Na<SUB>tur</SUB>)×845.813-6.231 (<I>r</I><SUP>2</SUP>=0.95), where Nafluid is the concentration of Na+ in the final fluid (mol/l) and Natur is the number of Na cations in the X-site of tourmaline. The equations are valid in the temperature range of 500–715 °C. Our experiments demonstrate that the occupancy of the X-site in combination with the changing concentrations of Al and Mg can be used to monitor changes in the fluid composition in equilibrium with a growing tourmaline crystal. Currently, this relation can be applied qualitatively to natural tourmaline to explain zoning in Na- and Al/(Al+Mg).
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Electronic Publication
Rights and permissions
About this article
Cite this article
v. Goerne, G., Franz, G. & Heinrich, W. Synthesis of tourmaline solid solutions in the system Na2O–MgO–Al2O3–SiO2–B2O3–H2O–HCl and the distribution of Na between tourmaline and fluid at 300 to 700 °C and 200 MPa. Contrib Mineral Petrol 141, 160–173 (2001). https://doi.org/10.1007/s004100100243
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s004100100243