Abstract
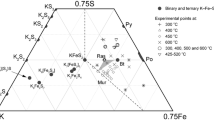
Detailed phase relations have been determined within the systems Fe2O3-MgO-TiO2 and FeO-MgO-TiO2. Experiments were performed over the temperature interval 1173–1473 K by equilibrating pelletized, fine-grained oxide mixtures in either inert calcia-stabilized zirconia pots (Fe2O3-MgO-TiO2 system) or evacuated silica tubes (FeO-MgO-TiO2 system). Equilibrium phase assemblages were determined by combined optical microscope, X-ray diffraction and EMP examination. Phase relations in the Fe2O3-MgO-TiO2 ternary are dominated by the instability of the M2O3 solid solution relative to the phase assemblage M3O4 + M3O5. A miscibility gap along the M2O3 binary also gives rise to two, 3-phase fields (α-M2O3 + M3O5 + M3O4 and α′-M2O3 + M3O5 + M3O4) separated by the M3O4 + M3O5 phase field. Phase relations in the FeO-MgO-TiO2 ternary were divided into two sub-systems. For the FeTiO3-MgTiO3-TiO2 sub-ternary, there is complete solid solution along the M2O3 and M3O5 binary joins at high temperature. At low temperatures (T < 1373 K) the M3O5 pseudobrookite solid solution decomposes to M2O3 + TiO2. Increasing the concentration of MgO in M3O5 phase results in a decrease in the temperature at which M3O5 becomes unstable and compositional tie lines linking M2O3 and TiO2 fan out, before the appearance of a three-phase region where M2O3, M3O5, and TiO2 coexist. Within the expanded FeO-MgO-TiO2 system, at temperatures above ∼1273 K there is a continuous solid solution along the M3O4 binary. At low temperatures (T < 1273 K) the Mg2TiO4 end-member breaks down to MgO and MgTiO3. The M3O4 phase shows significant non-stoichiometry, down to at least 1173 K. Fe2+-Mg partitioning data were obtained for coexisting M2O3-M3O5 and M2O3-M3O4 pairs in the FeO-MgO-TiO2 ternary. Assuming a regular solution mixing model for all phases, the M2O3 and M3O4 solid solutions were both found to exhibit moderate positive deviations from ideality (∼2600 J/mol), whereas the data for the M3O5 binary suggest close to ideal behaviour.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Received: 22 May 1998 / Accepted: 3 November 1998
Rights and permissions
About this article
Cite this article
Pownceby, M., Fisher-White, M. Phase equilibria in the systems Fe2O3-MgO-TiO2 and FeO-MgO-TiO2 between 1173 and 1473 K, and Fe2+-Mg mixing properties of ilmenite, ferrous-pseudobrookite and ulvöspinel solid solutions. Contrib Mineral Petrol 135, 198–211 (1999). https://doi.org/10.1007/s004100050506
Issue Date:
DOI: https://doi.org/10.1007/s004100050506