Abstract
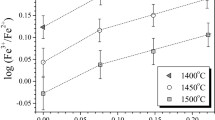
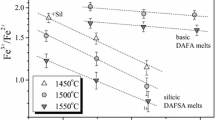
The effect of CaO, Na2O, and K2O on ferric/ferrous ratio in model multicomponent silicate melts was investigated in the temperature range 1450–1550 °C at 1-atm total pressure in air. It is demonstrated that the addition of these network modifier cations results in an increase of Fe3+/Fe2+ ratio. The influence of network modifier cations on the ferric/ferrous ratio increases in the order Ca < Na < K. Some old controversial conceptions concerning the effect of potassium on Fe3+/Fe2+ ratio in simple model liquids are critically evaluated. An empirical equation is proposed to predict the ferric/ferrous ratio in SiO2–TiO2–Al2O3–FeO–Fe2O3–MgO–CaO–Na2O–K2O–P2O5 melts at air conditions.








Similar content being viewed by others
References
Appora I, Eiler JM, Matthews A, Stolper EM (2003) Experimental determination of oxygen isotope fractionations between CO2 vapor and soda-melilite melt. Geochim Cosmochim Acta 67:459–471
Borisov A (2001) Loop technique: dynamic of metal/melt equilibration. Min Petrol 71:87–94
Borisov AA (2008) Experimental investigation of K and Na partitioning between miscible liquids. Petrology 16:552–564
Borisov AA (2009) Influence of SiO2 and Al2O3 on the activity coefficients of alkalis in melts: an experimental study. Petrology 17:579–590
Borisov A, McCammon C (2010) The effect of silica on ferric/ferrous ratio in silicate melts: an experimental investigation using Mössbauer spectroscopy. Am Miner 95:545–555
Borisov AA, Shapkin AI (1990) A new empirical equation relating Fe3+/Fe2+ in magmas to their composition, oxygen fugacity, and temperature. Geochem Int 27:111–116
Borisov A, Lahaye Y, Palme H (2006) The effect of sodium on the solubilities of metals in silicate melts. Am Mineral 91:762–771
Borisov A, Pack A, Kropf A, Palme H (2008) Partitioning of Na between olivine and melt: an experimental study with application to the formation of meteoritic Na2O-rich chondrule glass and refractory forsterite grains. Geochim Cosmochim Acta 72:5558–5573
Borisov A, Behrens H, Holtz F (2013) The effect of titanium and phosphorus on ferric/ferrous ratio in silicate melts: an experimental study. Contrib Min Petrol 166:1577–1591
Borisov A, Behrens H, Holtz F (2015) Effects of melt composition on Fe3+/Fe2+ in silicate melts: a step to model ferric/ferrous ratio in multicomponent systems. Contrib Min Petrol 169: Article 24
Bychkov AM, Borisov AA, Khramov DA, Urusov VS (1993) Change in the immediate environment of Fe atoms during the melting of minerals (Review). Geochem Int 30:1–25
Chuang H-C, Hwang W-S, Liu S-H (2009) Effects of basicity and FeO content on the softening and melting temperatures of the CaO–SiO2–MgO–Al2O3 slag system. Mat Trans 50:1448–1456
Cicconi MR, Giuli G, Ertel-Ingrisch W, Paris E, Dingwell DB (2015) The effect of the [Na/(Na + K)] ratio on Fe speciation in phonolitic glasses. Am Mineral 100:1610–1619
Cicconi MR, de Ligny D, Gall TM, Neuvill DR (2016) Ca neighbors from XANES spectroscopy: A tool to investigate structure, redox, and nucleation processes in silicate glasses, melts, and crystals. Am Mineral 101:1232–1235
Cottrell E, Kelley KA, Lanzirotti A, Fischer RA (2009) High-precision determination of iron oxidation state in silicate glasses using XANES. Chem Geol 268:167–179
Dickenson MP, Hess PC (1981) Redox equilibria and the structural role of iron in aluminosilicate melts. Contrib Mineral Petrol 78:352–357
Farges F, Lefrere Y, Rossano S, Berthereau A, Calas G, Brown GE Jr (2004) The effect of redox state on the local structural environment of iron in silicate glasses: a combined XAFS spectroscopy, molecular dynamics, and bond valence study. J Non-cryst Solids 344:176–188
Fudali RF (1965) Oxygen fugacities of basaltic and andesitic magmas. Geochim Cosmochim Acta 29:1063–1075
Hehlen B, Neuville DR (2015) Raman response of network modifier cations in alumino-silicate glasses. J Phys Chem B 119:4093–4098
Hirashima H, Yoshida T, Brückner R (1988) Redox equilibria and constitution of polyvalent ions in oxide melts and glasses. Glastech Ber 61: 283–291
Holmquist SB (1966) Ionic formulation of redox equilibria in glass melts. J Am Ceram Soc 49:228–229
Ishihara S (2004) The redox state of granitoids relative to tectonic setting and earth history: the magnetite-ilmenite series 30 years later. Geological Society of America Special Papers 389: 23–33
Jayasuriya KD, O’Neil HStC, Berry A, Campbell SJ (2004) A Mössbauer study of the oxidation state of Fe in silicate melts. Am Mineral 89:1597–1609
Kennedy GC (1948) Equilibrium between volatiles and iron oxides in igneous rocks. Am J Sci 246:529–549
Kilinc A, Carmichael ISE, Rivers ML, Sack RO (1983) The ferric-ferrous ratio of natural silicate liquids equilibrated in air. Contrib Mineral Petrol 83:136–140
Kjeldsen J, Smedskjaer MM, Mauro JC, Yue Y (2014) On the origin of the mixed alkali effect on indentation in silicate glasses. J Non-cryst Solids 406:22–26
Kress VC, Carmichael ISE (1989) The lime-iron-silicate melt system: redox and volume systematics. Geochim Cosmochim Acta 53:2883–2892
Kress VC, Carmichael ISE (1991) The compressibility of silicate liquids containing Fe2O3 and the effect of composition, temperature, oxygen fugacity and pressure on their redox states. Contrib Min Petrol 108:82–92
Lange RA, Carmichael ISE (1989) Ferric/ferrous equilibria in Na2O–FeO–Fe2O3–SiO2 melts: effects of analytical techniques on derived partial molar volumes. Geochim Cosmochim Acta 53:2195–2204
Le Losq C, Neuville DR (2013) Effect of the Na/K mixing on the structure and the rheology of tectosilicate silica-rich melts. Chem Geol 346:57–71
Moore G, Righter K, Carmichael ISE (1995) The effect of dissolved water on the oxidation state of iron in natural silicate liquids. Contrib Min Petrol 120:170–179
Mysen BO, Richet P (2005) Silicate glasses and melts, properties and structure. Elsevier, Amsterdam, p 544
Mysen BO, Toplis MJ (2007) Structural behavior of Al3+ in peralkaline, metaluminous, and peraluminous silicate melts and glasses at ambient pressure. Am Mineral 92:933–946
O’Neill HStC (2005) A method for controlling alkali-metal oxide activities in one-atmosphere experiments and its application to measuring the relative activity coefficients of NaO 0.5 in silicate melts. Am Mineral 90:497–501
Ottonello G, Moretti R, Marini L, Zuccolini MV (2001) Oxidation state of iron in silicate glasses and melts: a thermochemical model. Chem Geol 174:157–179
Park Y, Min DJ (2016) Sulfide Capacity of CaO–SiO2–FeO–Al2O3–MgOsatd. Slag ISIJ Int 56:520–526
Paul A, Douglas RW (1965) Ferrous-ferric equilibrium in binary alkali silicate glasses. Phys Chem Glasses 6:207–211
Putirka K (2016) Rates and styles of planetary cooling on Earth, Moon, Mars, and Vesta, using new models for oxygen fugacity, ferric-ferrous ratios, olivine-liquid Fe-Mg exchange, and mantle potential temperature. Am Mineral 101:819–840
Riebling EF (1966) Structure of Sodium Aluminosilicate Melts Containing at Least 50 mol % SiO2 at 1500 °C. J Chem Physics 44:2857–2865
Sack RO, Carmichael ISE, Rivers ML, Ghiorso MS (1980) Ferric-ferrous equilibria in natural silicate liquids at 1 bar. Contrib Mineral Petrol 75:369–376
Schreiber HD (1986) Redox processes in glass-forming melts. J Non-Cryst Solids 84:129–141
Schuessler JA, Botcharnikov RE, Behrens H, Misiti V, Freda C (2008) Oxidation state of iron in hydrous phono-tephritic melts. Am Mineral 93:1493–1504
Shand SJ (1927) Eruptive rocks; their genesis, composition, classification, and their relation to ore deposits, with a chapter on meteorites. 1st ed. 360 p. Thomas Murby and Co., London
Shibata K (1967) The oxygen partial pressure of the magma from Mihara Volcano, O-sima, Japan. Bull Chem Soc Jpn 40:830–834
Sisson TW, Grove TL (1993) Experimental investigation of the role of H2O in calc-alkaline differentiation and subduction zone magmatism. Contrib Min Petrol 113:143–166
Sukenaga S, Kanehashi K, Shibata H, Saito N, Nakashima K (2016) Structural role of alkali cations in calcium aluminosilicate glasses as examined using oxygen-17 solid-state nuclear magnetic resonance spectroscopy. Met Mat Trans 47B:2016–2177
Tangeman JA, Lange R, Forman L (2001) Ferric-ferrous equilibria in K2O–FeO–Fe2O3–SiO2 melts. Geochim Cosmochim Acta 65:1809–1819
Thornber CR, Roeder PL, Foster JR (1980) The effect of composition on the ferric-ferrous ratio in basaltic liquids at atmospheric pressure. Geochim Cosmochim Acta 44:525–532
Toplis MJ, Dingwell DB (2004) Shear viscosities of CaO–Al2O3–SiO2 and MgO–Al2O3–SiO2 liquids: implications for the structural role of aluminium and the degree of polymerisation of synthetic and natural aluminosilicate melts. Geochim Cosmochim Acta 68:5169–5188
Tsuchiyama A, Nagahara H, Hushiro I (1981) Volatilization of sodium from silicate melt spheres and its application to the formation of chondrules. Geochim Cosmochim Acta 45:1357–1367
Vercamer V, Lelong G, Hijiya H, Kondo Y, Galoisy L, Calas G (2015) Diluted Fe3+ in silicate glasses: structural effects of Fe-redox state and matrix composition. An optical absorption and X-band/Q-band EPR study. J Non-Cryst Solids 428:138–145
Webb SL, Banaszak M, Köhler U, Rausch S, Raschke G (2007) The viscosity of Na2O–CaO–Al2O3–SiO2 melts. Eur J Mineral 19:681–692
Wilke M, Farges F, Partzsch GM, Schmidt C, Behrens H (2007) Speciation of Fe in silicate glasses and melts by in-situ XANES spectroscopy. Am Mineral 92:44–56
Wilson AD (1960) The micro-determination of ferrous iron in silicate minerals by a volumetric and a colorimetric method. Analyst 85:823–827
Acknowledgements
The stay of AB in Hannover was funded by the German Science Foundation (DFG project Ho 1337/30-1). We thank Eric Wolff and Renat Almeev for the electron microprobe assistance and Florian Pohl for the help in the determination of ferric/ferrous ratios in glasses. We are grateful to editor Chris Ballhaus and three anonymous reviewers for their comments and suggestions which allowed us to improve the paper. This study was partly supported by Russian Science Foundation (Grant 14-17-00491).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Prof. Othmar Müntener.
Rights and permissions
About this article
Cite this article
Borisov, A., Behrens, H. & Holtz, F. Effects of strong network modifiers on Fe3+/Fe2+ in silicate melts: an experimental study. Contrib Mineral Petrol 172, 34 (2017). https://doi.org/10.1007/s00410-017-1337-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00410-017-1337-1