Abstract
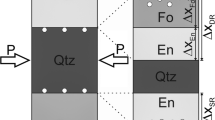
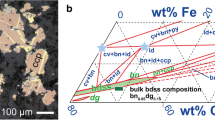
Reaction rims of dolomite (CaMg[CO3]2) were produced by solid-state reactions at the contacts of oriented calcite (CaCO3) and magnesite (MgCO3) single crystals at 400 MPa pressure, 750–850 °C temperature, and 3–146 h annealing time to determine the reaction kinetics. The dolomite reaction rims show two different microstructural domains. Elongated palisades of dolomite grew perpendicular into the MgCO3 interface with length ranging from about 6 to 41 µm. At the same time, a 5–71 µm wide rim of equiaxed granular dolomite grew at the contact with CaCO3. Platinum markers showed that the original interface is located at the boundary between the granular and palisade-forming dolomite. In addition to dolomite, a 12–80 µm thick magnesio-calcite layer formed between the dolomite reaction rims and the calcite single crystals. All reaction products show at least an axiotactic crystallographic relationship with respect to calcite reactant, while full topotaxy to calcite prevails within the granular dolomite and magnesio-calcite. Dolomite grains frequently exhibit growth twins characterized by a rotation of 180° around one of the \([11\bar{2}0]\) equivalent axis. From mass balance considerations, it is inferred that the reaction rim of dolomite grew by counter diffusion of MgO and CaO. Assuming an Arrhenius-type temperature dependence, activation energies for diffusion of CaO and MgO are E a (CaO) = 192 ± 54 kJ/mol and E a (MgO) = 198 ± 44 kJ/mol, respectively.










Similar content being viewed by others
References
Abart R, Petrishcheva E (2011) Thermodynamic model for reaction rim growth: interface reaction and diffusion control. Am J Sci 311:517–527. doi:10.2138/am.2011.3820
Abart R, Schmid R et al (2004) Silicon and oxygen self-diffusion in enstatite polycrystals: the Milke et al. (2001) rim growth experiment revisited. Contrib Miner Petrol 147:633–646. doi:10.1007/s00410-004-0596-9
Abart R, Petrishcheva E, Fischer FG, Svoboda J (2009) Thermodynamic model for diffusion controlled reaction rim growth in a binary system: application to the forsterite-enstatite-quartz system. Am J Sci 309:114–131. doi:10.2475/02.2009.02
Adams BL, Wright SI, Kunze K (1993) Orientation imaging: the emergence of a new microscopy. Met Trans 24A:819–831. doi:10.1007/BF02656503
Anderson TF (1972) Self-diffusion of carbon and oxygen in dolomite. J Geophys Res 77:857–861. doi:10.1029/JB077i005p00857
Ashworth JR, Sheplev VS, Bryxina NA, Kolobov VY, Reverdatto VV (1998) Diffusion controlled corona reaction and overstepping of equilibrium in a garnet granulite, Yenisey Ridge, Siberia. J Metam Geol 16:231–246. doi:10.111/j.1525-1314.1998.00134.x
Dohmen R, Milke R (2010) Diffusion in polycrystalline materials: grain boundaries, mathematical models, and experimental data. Rev Miner Geochem 72:921–970. doi:10.2138/rmg.2010.72.21
Farver J, Yund R (1996) Volume and grain boundary diffusion of calcium in natural and hot-pressed calcite aggregates. Contrib Miner Petrol 123:77–91
Fisher GW (1978) Rate laws in metamorphism. Geochim Cosmochim Acta 42:1035–1050. doi:10.1016/0016-7037(78)90292-2
Fisler DK, Cygan RT (1999) Diffusion of Ca and Mg in calcite. Am Miner 84:1392–1399
Gardés E, Heinrich W (2011) Growth of multilayered polycristalline reaction rims in the MgO-SiO2 system, part II: modelling. Contrib Miner Petrol 162:37–49. doi:10.1007/s00410-010-0581-4
Gardés E, Wunder B, Wirth R, Heinrich W (2011) Growth of multilayered polycrystalline reaction rims in the MgO-SiO2 system, part I: experiments. Contrib Miner Petrol 161:1–12. doi:10.1007/s00410-010-0517-z
Gardés E, Wunder B, Marquard K, Heinrich W (2012) The effect of water on intergranular mass transport: new insights from diffusion-controlled reaction rims in the MgO–SiO2 system. Contrib Miner Petrol 164:1–16. doi:10.1007/s00410-012-0721-0
Goldsmith JR, Heard HC (1961) Subsolidus phase relations in the system CaCO3–MgCO3. J Geol 69:45–74
Gottschalk M, Metz P (1992) The system calcite-dolomite: a model to calculate the Gibbs free energy of mixing on the basis of existing experimental data. N Jb Min Abh 164:29–55
Götze LC, Abart R, Rybacki E, Keller LM, Petrishcheva E, Dresen G (2010) Reaction rim growth in the system MgO–Al2O3–SiO2 under uniaxial stress. Miner Petrol 99:263–277. doi:10.1007/s00710-009-0080-3
Holland TJB, Powell R (1998) An internally consistent thermodynamic dataset for phases of petrologic interest. J Met Geol 16:309–343
Joachim B, Gardés E, Abart R, Heinrich W (2011) Experimental growth of åkermanite reaction rims between wollastonite and monticellite: evidence for volume diffusion control. Contrib Miner Petrol 161:389–399. doi:10.1007/s00410-010-0538-7
Joesten R (1977) Evolution of mineral assemblage zoning in diffusion metasomatism. Geochim Cosmochim Acta 41:649–670. doi:10.1016/0016-7037(77)90303-9
Keller LM, Abart R, Wirth R, Schmid DW, Kunze K (2006) Enhanced mass transfer through short circuit diffusion: growth of garnet reaction rims at eclogite facies conditions. Am Miner 91:1024–1038. doi:10.2138/am2006.2068
Keller LM, Wirth R, Rhede D, Kunze K, Abart R (2008a) Asymmetrically zoned reaction rims: assessment of grain boundary diffusivities and growth rates related to natural diffusion-controlled mineral reactions. J Metamorphic Geol 26:99–120. doi:10.1111/j.1525-1314.2007.00747.x
Keller LM, Wunder B, Rhede D, Wirth R (2008b) Component mobility at 900 °C and 18 kbar from experimentally grown coronas in a natural gabbro. Geochim Cosmochim Acta 72:4307–4322. doi:10.1016/j.gca.2008.05.054
Keller LM, Götze LC, Rybacki E, Dresen G, Abart R (2010) Enhancement of solid-state reaction rates by non-hydrostatic stress effects on polycrystalline diffusion kinetics. Am Miner 95:1399–1407. doi:10.2138/am.2010.3372
Kent AJR, Hutcheon ID, Ryerson FJ, Phinney DL (2001) The temperature of formation of carbonate in Martian meteorite ALH84001: constraints from cation diffusion. Geochim Cosmochim Acta 65:311–321. doi:10.1016/S0016-7037(00)00528-7
Lasaga AC (1983) Geospeedometry: an extension of geothermometry. In: Saxena SK (ed) Kinetics and equilibrium in mineral reactions. Springer, New York, pp 81–114
Milke R, Heinrich W (2002) Diffusion-controlled growth of wollastonite rims between quartz and calcite: comparison between nature and experiment. J Metamorph Geol 20:467–480. doi:10.1007/s00269-003-0304-8
Milke R, Wirth R (2003) The formation of columnar fiber texture in wollastonite rims by induced stress and implications for diffusion-controlled corona growth. Phys Chem Miner 30:230–242. doi:10.1007/s00269-003-0304-8
Milke R, Wiedenbeck M, Heinrich W (2001) Grain boundary diffusion of Si, Mg, and O in enstatite reaction rims: a SIMS study using isotopically doped reactants. Contrib Miner Petrol 142:15–26. doi:10.1007/s004100100277
Milke R, Abart R, Kunze K, Koch-Müller M, Schmid D, Ulmer P (2009) Matrix rheology effects on reaction rim growth I: evidence from orthopyroxene rim growth experiments. J Metamorphic Geol 27:71–82. doi:10.111/j.1525-1314.2008.00804.x
Milke R, Neusser G, Kolzer K, Wunder B (2013) Very little water is necessary to make a dry solid silicate system wet. Geology 41:247–250. doi:10.1130/G33674.1
Mrowec S (1980) Defects and diffusion in solids: an introduction. Elsevier, Amsterdem
Müller T, Cherniak D, Watson B (2012) Interdiffusion of divalent cations in carbonates: experimental measurements and implications for timescales of equilibration and retention of compositional signatures. Geochim Cosmochim Acta 84:90–103. doi:10.1016/j.gca.2012.01.011
Paterson MS (1970) A high-pressure temperature apparatus for rock deformation. Int J Rock Mech Min Sci 7:517–526. doi:10.1016/0148-9062(70)90004-5
Schmalzried H (1978) Reactivity and point defects of double oxides with emphasis on simple silicates. Phys Chem Miner 2:279–294. doi:10.1007/BF00308179
Watson EB, Price J (2002) Kinetics of the reaction MgO + Al2O3 → MgAl2O4 and Al–Mg interdiffusion in spinel at 1200 to 2000°C and 1.0 to 4.0 GPa. Geochim Cosmochim Acta 66:2123–2138. doi:10.1016/s0016-7037(02)00827-x
Acknowledgments
We are grateful to S. Gehrmann for sample preparation, M. Naumann for technical support with the Paterson apparatus and W. Heinrich for fruitful discussions. Two anonymous reviewers are thanked for their insightful comments. This work was funded by the Deutsche Forschungsgemeinschaft within the framework of FOR 741, project RY 103/1-1, which is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by H. Keppler.
Rights and permissions
About this article
Cite this article
Helpa, V., Rybacki, E., Abart, R. et al. Reaction kinetics of dolomite rim growth. Contrib Mineral Petrol 167, 1001 (2014). https://doi.org/10.1007/s00410-014-1001-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00410-014-1001-y