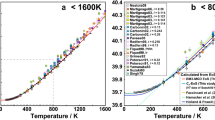
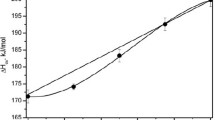
Abstract
We define and calibrate a new model of molar volume as a function of pressure, temperature, ordering state, and composition for spinels in the supersystem (Mg, Fe2+)(Al, Cr, Fe3+)2O4 − (Mg, Fe2+)2TiO4. We use 832 X-ray and neutron diffraction measurements performed on spinels at ambient and in situ high-P, T conditions to calibrate end-member equations of state and an excess volume model for this system. The effect on molar volume of cation ordering over the octahedral and tetrahedral sites is captured with linear dependence on Mg2+, Al3+, and Fe3+ site occupancy terms. We allow standard-state volumes and coefficients of thermal expansion of the end members to vary within their uncertainties during extraction of the mixing properties, in order to achieve the best fit. Published equations of state of the various spinel end members are analyzed to obtain optimal values of the bulk modulus and its pressure derivative, for each explicit end member. For any spinel composition in the supersystem, the model molar volume is obtained by adding excess volume and cation order-dependent terms to a linear combination of the five end-member volumes, estimated at pressure and temperature using the high-T Vinet equation of state. The preferred model has a total of 9 excess volume and order-dependent parameters and fits nearly all experiments to within 0.02 J/bar/mol, or better than 0.5 % in volume. The model is compared to the current MELTS spinel model with a demonstration of the impact of the model difference on the estimated spinel-garnet lherzolite transition pressure.










Similar content being viewed by others
References
Akimoto S (1954) Thermo-magnetic study of ferromagnetic minerals contained in igneous rocks. J Geomagn Geoelectr 6:1–14
Andreozzi GB, Lucchesi S (2002) Intersite distribution of Fe2+ and Mg in the spinel (sensu stricto)-hercynite series by single-crystal X-ray diffraction. Am Mineral 87:1113
Andreozzi GB, Princivalle F (2002) Kinetics of cation ordering in synthetic MgAl2O4 spinel. Am Mineral 87:838–844
Andreozzi GB, Princivalle F, Skogby H, Della Giusta A (2000) Cation ordering and structural variations with temperature in MgAl2O4 spinel: an X-ray single-crystal study. Am Mineral 85:1164–1171
Andreozzi GB, Lucchesi S, Skogby H, Della Giusta A (2001) Compositional dependence of cation distribution in some synthetic (Mg, Zn)(Al, Fe3+)2O4 spinels. Eur J Mineral 13:391–402
Antao SM, Hassan I, Parise JB (2005a) Cation ordering in magnesioferrite, MgFe2O4, to 982 °C using in situ synchrotron X-ray powder diffraction. Am Mineral 90:219–228
Antao SM, Hassan I, Crichton WA, Parise JB (2005b) Effects of high pressure and high temperature on cation ordering in magnesioferrite, MgFe2O4, using in situ synchrotron X- ray powder diffraction up to 1430 K and 6 GPa. Am Mineral 90:1500–1505
Asimow PD, Dixon JE, Langmuir CH (2004) A hydrous melting and fractionation model for mid-ocean ridge basalts: application to the Mid-Atlantic Ridge near the Azores. Geochem Geophys Geosyst 5. doi:10.1029/2003GC000568
Barnes SJ, Roeder PL (2001) The range of spinel compositions in terrestrial mafic and ultramafic rocks. J Petrol 42:2279–2302
Berman RG (1988) Internally-consistent thermodynamic data for minerals in the system Na2O-K2O-CaO-MgO-FeO-Fe2O3-Al2O3-SiO2-TiO2-H2O-CO2. J Petrol 89:168–183
Berman RG, Koziol AM (1991) Ternary excess properties of grossular-pyrope-almandine garnet and their influence in geothermobarometry. Am Mineral 76:1223–1231
Bhagavantam S (1955) Elastic properties of single crystals and polycrystalline aggregates. Proc Math Sci 41:72–90
Bosi F, Hålenius U, Andreozzi GB, Skogby H, Lucchesi S (2007) Structural refinement and crystal chemistry of Mn-doped spinel: a case for tetrahedrally coordinated Mn3+ in an oxygen-based structure. Am Mineral 92:27–33
Bosi F, Hålenius U, Skogby H (2009) Crystal chemistry of the magnetite-ulvöspinel series. Am Mineral 94:181–189
Bragg W (1915) The structure of magnetite and the spinels. Nature 95:561
Brey GP, Doroshev AM, Girnis AV, Turkin AI (1999) Garnet-spinel-olivine-orthopyroxene equilibria in the FeO-MgO-Al2O3-SiO2-Cr2O3 system: I. Composition and molar volumes of minerals. Eur J Mineral 11:599–617
Buddington AF, Lindsley DH (1964) Iron-titanium oxide minerals and synthetic equivalents. J Petrol 5:310–357
Callen HB, Harrison SE, Kriessman CJ (1956) Cation distributions in ferrospinels. Theoretical Phys Rev 103:851–856
Carbonin S, Russo U, Della Giusta A (1996) Cation distribution in some natural spinels from X-ray diffraction and Mössbauer spectroscopy. Mineral Mag 60:355–368
Carbonin S, Martignago F, Menegazzo G, Dal Negro A (2002) X-ray single-crystal study of spinels: in situ heating. Phys Chem Miner 29:503–514
Carraro A (2003) Crystal chemistry of Cr-spinels from a suite of spinel peridotite mantle xenoliths from the Predazzo Area (Dolomites, Northern Italy). Eur J Mineral 15:681–688
Connolly JAD (2009) The geodynamic equation of state: What and how. Geochem Geophys Geosys 10. doi:10.1029/2009GC002540
Della Giusta A, Carbonin S, Ottonello G (1996) Temperature-dependent disorder in a natural Mg-Al-Fe2 + -Fe3 + -spinel. Mineral Mag 60:603–616
Dick HJB, Bullen T (1984) Chromian spinel as a petrogenetic indicator in abyssal and alpine- type peridotites and spatially associated lavas. Contrib Mineral Petrol 86:54–76
Doraiswami MS (1947) Elastic constants of magnetite, pyrite and chromite. Proc Math Sci 25:413–416
Doroshev AM, Brey GP, Girnis AV, Turkin AI, Kogarko LN (1997) Pyrope-knorringite garnets in the Earth’s mantle: experiments in the MgO-Al2O3-SiO2-Cr2O3 system. Russ Geol Geophys 38:559–586
Downs RT, Hall-Wallace M (2003) The American Mineralogist crystal structure database. Am Mineral 88:247–250
Dunitz J, Orgel L (1957) Electronic properties of transition-metal oxides-II: cation distribution amongst octahedral and tetrahedral sites. J Phys Chem Solids 3:318–323
Efron B (1982) The jackknife, the bootstrap, and other resampling plans. Soc Ind Appl Math, Philadelphia
Fan D, Zhou W, Liu C, Liu Y, Jiang X, Wan F, Liu J, Li X, Xie H (2008) Thermal equation of state of natural chromium spinel up to 26.8 GPa and 628 K. J Mater Sci 43:5546–5550
Finger LW, Hazen RM, Hofmeister AM (1986) High-pressure crystal chemistry of spinel (MgAl2O4) and magnetite (Fe3O4): comparisons with silicate spinels. Phys Chem Miner 13:215–220
Fleet ME (1981) The structure of magnetite. Acta Crystallogr B37:917–920
Fleet ME (1984) The structure of magnetite: two annealed natural magnetites, Fe3.005O4 and Fe2.96Mg0.04O4. Acta Crystallogr C40:1491–1493
Gatta G, Kantor I, Boffa Ballaran T, Dubrovinsky L, McCammon C (2007) Effect of non-hydrostatic conditions on the elastic behaviour of magnetite: an in situ single-crystal X-ray diffraction study. Phys Chem Miner 34:627–635
Ghiorso MS (1990) Thermodynamic properties of hematite-ilmenite-geikielite solid solutions. Contrib Mineral Petrol 104:645–667
Ghiorso MS (2004a) An equation of state for silicate melts. I. Formulation of a general model. Am J Sci 304:637–678
Ghiorso MS (2004b) An equation of state for silicate melts. III. Analysis of stoichiometric liquids at elevated pressure: shock compression data, molecular dynamics simulations, and mineral fusion curves. Am J Sci 304:752–810
Ghiorso MS (2004c) An equation of state for silicate melts. IV. Calibration of a multicomponent mixing model to 40 GPa. Am J Sci 304:811–838
Ghiorso MS, Evans BW (2008) Thermodynamics of rhombohedral oxide solid solutions and a revision of the Fe-Ti two-oxide geothermometer and oxygen- barometer. Am J Sci 308:957–1039
Ghiorso MS, Kress VC (2004) An equation of state for silicate melts. II. Calibration of volumetric properties at 105 Pa. Am J Sci 304:679–751
Ghiorso MS, Sack RO (1991) Fe-Ti oxide geothermometry: thermodynamic formulation and the estimation of intensive variables in silicic magmas. Contrib Mineral Petrol 108:485–510
Ghiorso MS, Sack RO (1995) Chemical mass transfer in magmatic processes IV. A revised and internally consistent thermodynamic model for the interpolation and extrapolation of liquid-solid equilibria in magmatic systems at elevated temperatures and pressures. Contrib Mineral Petrol 119:197–212
Ghiorso MS, Hirschmann MM, Reiners PW, Kress III VC (2002) The pMELTS: a revision of MELTS for improved calculation of phase relations and major element partitioning related to partial melting of the mantle to 3 GPa. Geochem Geophys Geosyst 3. doi:10.1029/2001GC000217
Ghiorso, MS, Hirschmann, MM, Grove, TL (2007) xMELTS: A thermodynamic model for the estimation of magmatic phase relations over the pressure range 0–30 GPa and at temperatures up to 2500 °C. Eos Trans Am Geophys Union 88(52), Fall Meet Suppl Abstr V31C-0608
Girnis A, Brey G, Doroshev A, Turkin A, Simon N (2003) The system MgO-Al2O3-Cr2O3 revisited: reanalysis of Doroshev et al’.s (1997) experiments and new experiments. Eur J Mineral 15:953–964
Golla-Schindler U, O’Neill HStC, Putnis A (2005) Direct observation of spinodal decomposition in the magnetite-hercynite system by susceptibility measurements and transmission electron microscopy. Am Mineral 90:1278–1283
Haavik C, Stølen S, Fjellvåg H, Hanfland M, Häusermann D (2000) Equation of state of magnetite and its high-pressure modification: thermodynamics of the Fe-O system at high pressure. Am Mineral 85:514–523
Haggerty S (1971) Compositional variations in lunar spinels. Nat Phys Sci 233:156–160
Hamecher EA, Antoshechkina PM, Ghiorso MS, Asimow PD (2009) Thermodynamic calibration of Cr-Al exchange equilibria for garnet and spinel. Eos Trans Am Geophys Union 90(52), Fall Meet Suppl Abstr V31D-2056
Harrison RJ, Redfern SAT, O’Neill HStC (1998) The temperature dependence of the cation distribution in synthetic hercynite (FeAl~2O~4) from in situ neutron structure refinements. Am Mineral 83:1092–1099
Hazen RM, Navrotsky A (1996) Effects of pressure on order-disorder reactions. Am Mineral 81:1021–1035
Hill RJ (1984) X-ray powder diffraction profile refinement of synthetic hercynite. Am Mineral 69:937–942
Hirschmann MM, Ghiorso MS, Davis FA, Gordon SM, Mukherjee S, Grove TL, Krawczynski M, Medard E, Till CB (2008) Library of experimental phase relations (LEPR): a database and web portal for experimental magmatic phase equilibria data. Geochem Geophys Geosyst 9. doi:10.1029/2007GC001894
Holland TJB, Powell R (1990) An enlarged and updated internally consistent thermodynamic dataset with uncertainties and correlations: the system K2O–Na2O–CaO–MgO–MnO– FeO–Fe2O3–Al2O3–TiO2–SiO2–C–H2–O2. J Metamorph Geol 8:89–124
Holland TJB, Powell R (1998) An internally consistent thermodynamic data set for phases of petrological interest. J Metamorph Geol 16:309–343
Holland TJB, Powell R (2011) An improved and extended internally consistent thermodynamic dataset for phases of petrological interest, involving a new equation of state for solids. J Metamorph Geol 29:333–383
Irifune T, Ohtani E, Kumazawa M (1982) Stability field of knorringite Mg3Cr2Si3O12 at high pressure and its implication to the occurrence of Cr-rich pyrope in the upper mantle. Phys Earth Planet Inter 27:263–272
Ishii M, Nakahira M, Yamanaka T (1972) Infrared absorption spectra and cation distributions in (Mn, Fe)3O4. Solid State Commun 11:209–212
Ishii M, Hiraishi J, Yamanaka T (1982) Structure and lattice vibrations of Mg-Al spinel solid solution. Phys Chem Miner 8:64–68
Kessel R, Beckett JR, Stolper EM (2003) Experimental determination of the activity of chromite in multicomponent spinels. Geochim Cosmochim Acta 67:3033–3044
Klemme S (2004) The influence of Cr on the garnet–spinel transition in the Earth’s mantle: experiments in the system MgO–Cr2O3–SiO2 and thermodynamic modelling. Lithos 77:639–646
Klemme S, Ivanic TJ, Connolly JAD, Harte B (2009) Thermodynamic modelling of Cr-bearing garnets with implications for diamond inclusions and peridotite xenoliths. Lithos 112:986–991
Larsson L, O’Neill HStC, Annersten H (1994) Crystal chemistry of synthetic hercynite (FeAl2O4) from XRD structural refinements and Mössbauer spectroscopy. Eur J Mineral 6:39–51
Lavina B, Koneva A, Della Giusta A (2003) Cation distribution and cooling rates of Cr- substituted Mg-Al spinel from the Olkhon metamorphic complex, Russia. Eur J Mineral 15:435–441
Lavina B, Princivalle F, Della Giusta A (2005) Controlled time-temperature oxidation reaction in a synthetic Mg-hercynite. Phys Chem Miner 32:83–88
Lavina B, Cesare B, Alvarez-Valero AM, Uchida H, Downs RT, Koneva A, Dera P (2009) Closure temperatures of intracrystalline ordering in anatectic and metamorphic hercynite, Fe2+Al2O4. Am Mineral 94:657–665
Lenaz D, Princivalle F (2005) The crystal chemistry of detrital chromian spinel from the southeastern Alps and outer Dinarides: the discrimination of supplies from areas of similar tectonic setting? Can Mineral 43:1305–1314
Lenaz D, Skogby H, Princivalle F, Hålenius U (2004) Structural changes and valence states in the MgCr2O4-FeCr2O4 solid solution series. Phys Chem Miner 31:633–642
Lenaz D, Braidotti R, Princivalle F, Garuti G, Zaccarini F (2007) Crystal chemistry and structural refinement of chromites from different chromitite layers and xenoliths of the Bushveld Complex. Eur J Mineral 19:599–609
Lenaz D, Logvinova AM, Princivalle F, Sobolev NV (2009) Structural parameters of chromite included in diamond and kimberlites from Siberia: a new tool for discriminating ultramafic source. Am Mineral 94:1067–1070
Levy D, Artioli G (1998) Thermal expansion of chromites and zinc spinels. Mater Sci Forum 278–281:390–395
Levy D, Pavese A, Hanfland M (2003) Synthetic MgAl2O4 (spinel) at high-pressure conditions (0.0001–30 GPa): a synchrotron X-ray powder diffraction study. Am Mineral 88:93–98
Levy D, Diella V, Dapiaggi M, Sani A, Gemmi M, Pavese A (2004) Equation of state, structural behaviour and phase diagram of synthetic MgFe2O4, as a function of pressure and temperature. Phys Chem Miner 31:122–129
Lindsley DH (1965) Iron-titanium oxides. Carnegie Inst Year B 64:144–148
Lucchesi S, Amoriello M, Della Giusta A (1998) Crystal chemistry of spinels from xenoliths of the Alban Hills volcanic region. Eur J Mineral 10:473–482
Martignago F, Dal Negro A, Carbonin S (2003) How Cr3+ and Fe3+ affect Mg–Al order–disorder transformation at high temperature in natural spinels. Phys Chem Miner 30:401–408
Martignago F, Andreozzi G, Dal Negro A (2006) Thermodynamics and kinetics of cation ordering in natural and synthetic Mg(Al, Fe3+)2O4 spinels from in situ high-temperature X-ray diffraction. Am Mineral 91:306–312
Mattioli GS, Wood BJ, Carmichael ISE (1987) Ternary-spinel volumes in the system MgAl2O4–Fe3O4–γFe8/3O4: implications for the effect of P on intrinsic fo2 measurements of mantle- xenolith spinels. Am Mineral 72:468–480
Méducin F, Redfern SAT, Le Godec Y, Stone HJ, Tucker MG, Dove MT, Marshall WG (2004) Study of cation order-disorder in MgAl2O4 spinel by in situ neutron diffraction up to 1600 K and 3.2 GPa. Am Mineral 89:981–986
Menegazzo G, Carbonin S (1998) Oxidation mechanisms in Al-Mg-Fe spinels. A second stage: α-Fe2O3 exsolution. Phys Chem Miner 25:541–547
Millard RL, Peterson RC, Hunter BK (1995) Study of the cubic to tetragonal transition in Mg2TiO4 and Zn2TiO4 spinels by 17O MAS NMR and Rietveld refinement of X-ray diffraction data. Am Mineral 80:885–896
Muan A, Hauck J, Löfall T (1972) Equilibrium studies with a bearing on lunar rocks. In: Proceedings of the Third Lunar Science Conference (Suppl 3). Geochim Cosmochim Acta 1:185–196
Nakagiri N, Manghnani MH, Ming LC, Kimura S (1986) Crystal structure of magnetite under pressure. Phys Chem Miner 13:238–244
Nakatsuka A, Ueno H, Nakayama N, Mizota T, Maekawa H (2004) Single-crystal X-ray diffraction study of cation distribution in MgAl2O4–MgFe2O4 spinel solid solution. Phys Chem Miner 31:278–287
Nell J, Wood BJ (1989) Thermodynamic properties in a multicomponent solid solution involving cation disorder: Fe3O4–MgFe2O4–FeAl2O4–MgAl2O4 spinels. Am Mineral 74:1000–1015
Nestola F, Ballaran T, Balic-Zunic T, Princivalle F, Secco L, Dal Negro A (2007) Comparative compressibility and structural behavior of spinel MgAl2O4 at high pressures: the independency on the degree of cation order. Am Mineral 92:1838–1843
O’Neill HStC, Navrotsky A (1983) Simple spinels: crystallographic parameters, cation radii, lattice energies, and cation distribution. Am Mineral 68:181–194
O’Neill HStC, Navrotsky A (1984) Cations distributions and thermodynamic properties of binary spinel solid solutions. Am Mineral 69:733–753
Oka Y, Steinke P, Chatterjee ND (1984) Thermodynamic mixing properties of Mg(Al, Cr)2O4 spinel crystalline solution at high temperatures and pressures. Contrib Mineral Petrol 87:196–204
O’Neill HStC, Dollase WA (1994) Crystal structures and cation distributions in simple spinels from powder XRD structural refinements: MgCr2O4, ZnCr2O4, Fe3O4, and the temperature dependence of the cation distribution in ZnAl2O4. Phys Chem Miner 20:541–555
O’Neill HStC, Annersten H, Virgo D (1992) The temperature dependence of the cation distribution in magnesioferrite (MgFe2O4) from powder XRD structural refinements and Mössbauer spectroscopy. Am Mineral 77:725–740
O’Neill HStC, Redfern S, Kesson S, Short S (2003) An in situ neutron diffraction study of cation disordering in synthetic qandilite Mg2TiO4 at high temperatures. Am Mineral 88:860–865
Pascal ML, Fonteilles M, Boudouma O, Principe C (2011) Qandilite from Vesuvius skarn ejecta: conditions of formation and miscibility gap in the ternary spinel—qandilite—magnesioferrite. Can Mineral 49:459–485
Passerini L (1930) Richerche sugli spinelli. II. I composti. CuAl2O4, MgAl2O4, MgFe2O4, ZnAl2O4, ZnCr2O4, ZnFe2O4, MnFe2O4. Gazz Chim Ital 60:389–399
Peterson RC, Lager GA, Hitterman RL (1991) A time-of-flight neutron powder diffraction study of MgAl2O4 at temperatures up to 1273 K. Am Mineral 76:1455–1458
Powell R, Holland TJB (1985) An internally consistent thermodynamic dataset with uncertainties and correlations: 1. Methods and a worked example. J Metamorph Geol 3:327–342
Powell R, Holland TJB, Worley B (1998) Calculating phase diagrams involving solid solutions via non-linear equations, with examples using THERMOCALC. J Metamorph Geol 16:577–588
Princivalle F, Della Giusta A, De Min A, Piccirillo E (1999) Crystal chemistry and significance of cation ordering in Mg-Al rich spinels from high-grade hornfels (Predazzo-Monzoni, NE Italy). Mineral Mag 63:257–262
Princivalle F, Martignago F, Del Negro A (2006) Kinetics of cation ordering in natural Mg(Al, Cr3+)2O4 spinels. Am Mineral 91:313–318
Redfern SAT, Harrison RJ, O’Neill HStC, Wood DRR (1999) Thermodynamics and kinetics of cation ordering in MgAl2O4 spinel up to 1600 °C from in situ neutron diffraction. Am Mineral 84:299–310
Reichmann HJ, Jacobsen SD (2004) High-pressure elasticity of a natural magnetite crystal. Am Mineral 89:1061–1066
Robbins M, Wertheim GK, Sherwood RC, Buchanan DNE (1971) Magnetic properties and site distributions in the system FeCr2O4–Fe3O4 (Fe2+Cr2 – xFe 3+x O4). J Phys Chem Solids 32:717–729
Robie RA, Hemingway BS, Fisher JR (1979) Thermodynamic properties of minerals and related substances at 298.15 K and 1 bar (1e5 Pa) pressures and at higher temperatures. US Geol Surv Bull 1452:1–456
Sack RO (1982) Spinels as petrogenetic indicators: activity-composition relations at low pressures. Contrib Mineral Petrol 79:169–186
Sack RO, Ghiorso MS (1991a) An internally consistent model for the thermodynamic properties of Fe-Mg-titanomagnetite-aluminate spinels. Contrib Mineral Petrol 106:474–505
Sack RO, Ghiorso MS (1991b) Chromian spinels as petrogenetic indicators: thermodynamics and petrological applications. Am Mineral 76:827–847
Sack RO, Ghiorso MS (1994a) Thermodynamics of multi component pyroxenes: iI. Phase relations in the quadrilateral. Contrib Mineral Petrol 116:287–300
Sack RO, Ghiorso MS (1994b) Thermodynamics of multicomponent pyroxenes: III. Calibration of Fe2+(Mg)-1, TiAl2(MgSi2)-1, TiFe2 3+(MgSi2)-1, AlFe3+(MgSi)-1, NaAl(CaMg)-1, Al2(MgSi)-1 and Ca(Mg)-1 exchange reactions between pyroxenes and silicate melts. Contrib Mineral Petrol 118:271–296
Schwarz G (1978) Estimating the dimension of a model. Ann Stat 6:461–464
Sedler IK, Feenstra A, Peters T (1994) An X-ray powder diffraction study of synthetic (Fe, Mn)2TiO4 spinel. Eur J Mineral 6:873–885
Smith PM, Asimow PD (2005) Adiabat_1ph: a new public front-end to the MELTS, pMELTS, and pHMELTS models. Geochem Geophys Geosyst 6. doi:10.1029/2004GC000816
Stout M, Bayliss P (1980) Crystal structure of two ferrian ulvöspinels from British Columbia. Can Mineral 18:339–341
Taberna PL, Mitra S, Poizot P, Simon P, Tarascon J-M (2006) High rate capabilities Fe3O4- based Cu nano-architectured electrodes for lithium-ion battery applications. Nat Mater 5:567–573
Tabira Y, Withers RL (1999) Cation ordering in NiAl2O4 spinel by a 111 systematic row CBED technique. Phys Chem Miner 27:112–118
Verwey E, Heilmann E (1947) Physical properties and cation arrangement of oxides with spinel structures I. Cation arrangement in spinels. J Chem Phys 15:174–180
Waerenborgh JC, Figueiredo MO, Cabral JMP, Pereira LCJ (1994) Powder XRD structure refinements and 57Fe Mössbauer effect study of synthetic Zn1-xFexAl2O4 (0 < x ≤ 1) spinels annealed at different temperatures. Phys Chem Miner 21:460–468
Wang H, Simmons G (1972) Elasticity of some mantle crystal structures 1. Pleonaste and hercynite spinel. J Geophys Res 77:4379–4392
Wechsler BA, Von Dreele RB (1989) Structure refinements of Mg2TiO4, MgTiO3 and MgTi2O5 by time-of-flight neutron powder diffraction. Acta Crystallogr B45:542–549
Wechsler BA, Lindsley D, Prewitt C (1984) Crystal structure and cation distribution in titanomagnetites (Fe3-xTixO4). Am Mineral 69:754–770
Woodland AB, Bauer M, Ballaran TB, Hanrahan M (2009) Crystal chemistry of Fe3 2+Cr2Si3O12 – Fe3 2+Fe2 3+Si3O12 garnet solid solutions and related spinels. Am Mineral 94:359–366
Workman RK, Hart SR (2005) Major and trace element composition of the depleted MORB mantle (DMM). Earth Planet Sci Lett 231:53–72
Yamanaka T, Shimazu H, Ota K (2001) Electric conductivity of Fe2SiO4–Fe3O4 spinel solid solutions. Phys Chem Miner 28:110–118
Yang Z, Xia G-G, Li X-H, Stevenson JW (2007) (Mn, Co)3O4 spinel coatings on ferritic stainless steels for SOFC interconnect applications. Int J Hydrogen Energy 32:3648–3654
Zhao Y, Zhang Y, Bi C, Guo L (1998) The discovery of magnesioferrite from Au(Fe, Cu) magnesian skarn deposits and study of the magnesioferrite-magnesiomagnetite series. Acta Geol Sin 74:382–391
Acknowledgments
We wish to thank Peter Luffi for identifying the garnet solid solution error in the original MELTS code, Ashley Nagle for pointing out the anomalously low spinel–garnet transition pressures obtained when the corrected garnet model is used, and Aaron Wolf for helpful discussions regarding statistical analysis. Comments by Associate Editor Jon Blundy are greatly appreciated, as are the reviews of two anonymous reviewers. This work was supported by the National Science Foundation and the American Recovery and Reinvestment Act through award 0838244.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by J. Blundy.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hamecher, E.A., Antoshechkina, P.M., Ghiorso, M.S. et al. The molar volume of FeO–MgO–Fe2O3–Cr2O3–Al2O3–TiO2 spinels. Contrib Mineral Petrol 165, 25–43 (2013). https://doi.org/10.1007/s00410-012-0790-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00410-012-0790-0