Abstract
Purpose
We aimed to examine the correlation between clinical characteristics and the pathogenic gene variants in patients with Primary Ciliary Dyskinesia (PCD).
Methods
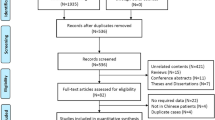
We conducted a retrospective single-center study in patients with PCD followed at the University Hospitals Leuven. We included patients with genetically confirmed PCD and described their genotype, data from ultrastructural ciliary evaluation and clinical characteristics. Genotype/phenotype correlations were studied in patients with the most frequently involved genes.
Results
We enrolled 74 patients with a median age of 25.58 years. The most frequently involved genes were DNAH11 (n = 23) and DNAH5 (n = 19). The most frequent types of pathogenic variants were missense (n = 42) and frameshift variants (n = 36) and most patients had compound heterozygous variants (n = 44). Ciliary ultrastructure (p < 0.001), situs (p = 0.015) and age at diagnosis (median 9.50 vs 4.71 years, p = 0.037) differed between DNAH11 and DNAH5. When correcting for situs this difference in age at diagnosis was no longer significant (p = 0.973). Patients with situs inversus were diagnosed earlier (p = 0.031). Respiratory tract microbiology (p = 0.161), lung function (cross-sectional, p = 0.829 and longitudinal, p = 0.329) and chest CT abnormalities (p = 0.202) were not significantly different between DNAH11 and DNAH5 variants.
Conclusion
This study suggests a genotype–phenotype correlation for some of the evaluated clinical characteristics of the two most frequently involved genes in this study, namely DNAH11 and DNAH5.




Similar content being viewed by others
Data Availability
No datasets were generated or analysed during the current study.
References
Lucas JS, Walker WT, Kuehni CE, Lazor R (2011) Primary ciliary dyskinesia. Orphan lung diseases European respiratory monograph, Latimer Trend & Co. Ltd, Plymouth, pp 201–217
Knowles MR, Zariwala M, Leigh M (2016) Primary ciliary dyskinesia. Clin Chest Med 37(3):449–461
Hornef N, Olbrich H, Horvath J, Zariwala MA, Fliegauf M, Loges NT et al (2006) DNAH5 mutations are a common cause of primary ciliary dyskinesia with outer dynein arm defects. Am J Respir Crit Care Med 174(2):120–126
Djakow J, Svobodová T, Hrach K, Uhlík J, Cinek O, Pohunek P (2012) Effectiveness of sequencing selected exons of DNAH5 and DNAI1 in diagnosis of primary ciliary dyskinesia. Pediatr Pulmonol 47(9):864–875
Kim RH, Hall DA, Cutz E, Knowles MR, Nelligan KA, Nykamp K et al (2014) The role of molecular genetic analysis in the diagnosis of primary ciliary dyskinesia. Ann Am Thoracic Soc 11(3):351–359
Knowles MR, Leigh MW, Carson JL, Davis SD, Dell SD, Ferkol TW et al (2012) Mutations of DNAH11 in patients with primary ciliary dyskinesia with normal ciliary ultrastructure. Thorax 67(5):433–441
Vanaken GJ, Bassinet L, Boon M, Mani R, Honore I, Papon JF et al (2017) Infertility in an adult cohort with primary ciliary dyskinesia: phenotype–gene association. Eur Respir J 50(5):1700314
Shapiro AJ, Davis SD, Polineni D, Manion M, Rosenfeld M, Dell SD et al (2018) Diagnosis of primary ciliary dyskinesia. An official American Thoracic Society clinical practice guideline. Am J Respir Crit Care Med 197(12):24–39
Lucas JS, Barbato A, Collins SA, Goutaki M, Behan L, Caudri D et al (2017) European Respiratory Society guidelines for the diagnosis of primary ciliary dyskinesia. Eur Respir J 49(1):1601090
Wallmeier J, Nielsen KG, Kuehni CE, Lucas JS, Leigh MW, Zariwala MA, Omran H (2020) Motile ciliopathies. Nat Rev Dis Primers 6(1):1–29
Mirra V, Werner C, Santamaria F (2017) Primary ciliary dyskinesia: an update on clinical aspects, genetics, diagnosis, and future treatment strategies. Front Pediatr 5:135
Bhatt R, Carr SB, Hogg C (2020) How insignificant are genetic variants of unknown significance in Primary Ciliary Dyskinesia. Eur Respir J 56(Suppl. 64):325
Shah A, Shoemark A, MacNeill SJ, Bhaludin B, Rogers A, Bilton D et al (2016) A longitudinal study characterising a large adult primary ciliary dyskinesia population. Eur Respir J 48(2):441–450
Pifferi M, Bush A, Mariani F, Piras M, Michelucci A, Cangiotti A et al (2020) Lung function longitudinal study by phenotype and genotype in primary ciliary dyskinesia. Chest 158(1):117–120
Raidt J, Wallmeier J, Hjeij R, Onnebrink JG, Pennekamp P, Loges NT et al (2014) Ciliary beat pattern and frequency in genetic variants of primary ciliary dyskinesia. Eur Respir J 44(6):1579–1588
Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J et al (2015) Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med 17(5):405–423
Centers for Disease Control and Prevention (2000) CDC growth charts for the United States: methods and development. Vital Health Stat 11(246):1–190
Shoemark A, Boon M, Brochhausen C, Bukowy-Bieryllo Z, De Santi MM, Goggin P et al (2020) International consensus guideline for reporting transmission electron microscopy results in the diagnosis of primary ciliary dyskinesia (BEAT PCD TEM Criteria). Eur Respir J 55(4):1900725
Guillien A, Soumagne T, Regnard J, Degano B (2018) The new reference equations of the Global Lung function Initiative (GLI) for pulmonary function tests. Rev Mal Respir 35(10):1020–1027
Lucas JS, Davis SD, Omran H, Shoemark A (2020) Primary ciliary dyskinesia in the genomics age. Lancet Respir Med 8(2):202–216
Shapiro AJ, Leigh MW (2017) Value of transmission electron microscopy for primary ciliary dyskinesia diagnosis in the era of molecular medicine: genetic defects with normal and non-diagnostic ciliary ultrastructure. Ultrastruct Pathol 41(6):373–385
Boon M, Smits A, Cuppens H, Jaspers M, Proesmans M, Dupont LJ et al (2014) Primary ciliary dyskinesia: critical evaluation of clinical symptoms and diagnosis in patients with normal and abnormal ultrastructure. Orphanet J Rare Dis 9:1–10
Barber AT, Shapiro AJ, Davis SD, Ferkol TW, Atkinson JJ, Sagel SD et al (2022) Laterality defects in primary ciliary dyskinesia: relationship to ultrastructural defect or genotype. Ann Am Thoracic Soc 20(3):397–405
Alanin MC, Nielsen KG, von Buchwald C, Skov M, Aanaes K, Høiby N, Johansen HK (2015) A longitudinal study of lung bacterial pathogens in patients with primary ciliary dyskinesia. Clin Microbiol Infect 21(12):1093-e1
Halbeisen FS, Pedersen ES, Goutaki M, Spycher BD, Amirav I, Boon M et al (2022) Lung function from school age to adulthood in primary ciliary dyskinesia. Eur Respir J 60(4):2101918
Paff T, Omran H, Nielsen KG, Haarman EG (2021) Current and future treatments in primary ciliary dyskinesia. Int J Mol Sci 22(18):9834
Ostrowski LE, Yin W, Patel M, Sechelski J, Rogers T, Burns K et al (2014) Restoring ciliary function to differentiated primary ciliary dyskinesia cells with a lentiviral vector. Gene Ther 21(3):253–261
McIntyre JC, Davis EE, Joiner A, Williams CL, Tsai IC, Jenkins PM et al (2012) Gene therapy rescues cilia defects and restores olfactory function in a mammalian ciliopathy model. Nat Med 18(9):1423–1428
Lai M, Pifferi M, Bush A, Piras M, Michelucci A, Di Cicco M et al (2016) Gene editing of DNAH11 restores normal cilia motility in primary ciliary dyskinesia. J Med Genet 53(4):242–249
Mani R, Gomes M, González AR, Hogg C, Morris-Rosendahl D, Maitre B et al (2021) Development and first results of the BEAT-PCD international Primary Ciliary Dyskinesia gene variant database: CiliaVar. Eur Respir J 58:PA3458
Raidt J, Maitre B, Pennekamp P, Altenburg J, Anagnostopoulou P, Armengot M et al (2022) The disease-specific clinical trial network for primary ciliary dyskinesia: PCD-CTN. ERJ Open Res. https://doi.org/10.1183/23120541.00139-2022
Acknowledgements
Mieke Boon participates in the European Reference Network for Rare Respiratory Diseases (ERN-LUNG) (739546) and in the “BEAT-PCD clinical research collaboration, supported by the European Respiratory Society.”
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
Noelia Rodriguez Mier: data curation, formal analysis, investigation, methodology, writing. Martine Jaspers: data curation and methodology. Evelien Van Hoof: data curation and methodology. Mark Jorissen: review and editing. Natalie Lorent: validation, review and editing. Marijke Proesmans: validation, review and editing. François Vermeulen: validation, review and editing. Jeroen Breckpot: validation, review and editing. Mieke Boon: conceptualization, methodology, formal analysis, investigation, supervision, validation, writing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Rodriguez Mier, N., Jaspers, M., Van Hoof, E. et al. Genetic Spectrum and Clinical Characteristics of Patients with Primary Ciliary Dyskinesia: a Belgian Single Center Study. Lung (2024). https://doi.org/10.1007/s00408-024-00696-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00408-024-00696-0