Abstract
Objective
Endogenous adenosine 5′-monophosphate (AMP), acetylcholine (ACh), and histamine (HA) are known to be important in bronchial contraction, but their clinical relevance to asthma is poorly understood. We aimed to quantify endogenous AMP, ACh, and HA in induced sputum samples and explore their relationships with asthma control and exacerbations.
Methods
20 healthy subjects and 112 asthmatics underwent clinical assessment, sputum induction, and blood sampling. The level of asthma control was determined by the asthma control test (ACT) questionnaire. Asthma exacerbation was evaluated according to the criteria of the American Thoracic Society/European Respiratory Society. Levels of AMP, ACh, and HA in sputum were measured by liquid chromatography coupled to tandem mass spectrometry. IL-β, IL-4, IL-5, IL-6, IL-8, IL-13, IL-17A, TNF-α, IFN-γ, and macrophage-derived chemokine (MDC) were also measured.
Results
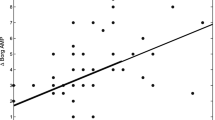
Compared to healthy controls, asthmatics had higher levels of HA, lower levels of ACh, and similar levels of AMP in induced sputum samples. Compared to controlled asthma (n = 54), uncontrolled asthma (n = 58) showed higher AMP levels (P = 0.002), but similar HA and ACh levels. AMP was negatively correlated with ACT scores (r = − 0.348) and asthma quality of life questionnaire scores (r = − 0.188) and positively correlated with blood monocytes percentage (r = 0.195), sputum MDC (r = 0.214), and IL-6 levels (r = 0.196). Furthermore, AMP was associated with an increased risk of exacerbations in the preceding year.
Conclusion
Endogenous AMP, but not ACh or HA, was associated with asthma control, quality of life, and exacerbations in the previous year, which indicates that AMP could be a clinically useful biomarker of asthma.




Similar content being viewed by others
Abbreviations
- AHR:
-
Airway hyper responsiveness
- ACh:
-
Acetylcholine
- AMP:
-
Adenosine 5′-monophosphate
- AChE:
-
Acetylcholinesterase
- ACT:
-
Asthma control test
- AQLQ:
-
Asthma quality of life questionnaire
- ASAN:
-
Australasian severe asthma network
- ATS:
-
American thoracic society
- BMI:
-
Body mass index
- CA:
-
Controlled asthma
- CRF:
-
Case report form
- CI:
-
Confidence interval
- COL4A3:
-
Collagen type 4 alpha 3
- ERS:
-
European respiratory society.
- EPA:
-
Exacerbation-prone asthma
- FEV1 :
-
Forced expiratory volume in 1 s
- FVC:
-
Forced vital capacity
- FT-ICR-MS:
-
Fourier transform ion cyclotron resonance mass spectrometry
- FeNO:
-
Fractional exhaled nitric oxide
- GINA:
-
Global initiative for asthma
- HA:
-
Histamine
- HADS:
-
Hospital anxiety and depression scale
- HADS-A:
-
Hospital anxiety and depression scale-anxiety
- HADS-D:
-
Hospital anxiety and depression scale-depression
- IL:
-
Interleukin
- IFN:
-
Interferon
- ICS:
-
Inhaled corticosteroid
- LC–MS/MS:
-
Liquid chromatography coupled to tandem mass spectrometry
- LLD:
-
Lower limit of detection
- IRR:
-
Incidence rate ratio
- MDC:
-
Macrophage-derived chemokine
- N:
-
Number
- OR:
-
Odds ratio
- Q:
-
Quartile
- SAWD:
-
Severe asthma web-based database
- SPT:
-
Skin prick tests
- SD:
-
Standard error
- SEM:
-
Standard error of the mean
- TNF:
-
Tumor necrosis factor
- TGF:
-
Transforming growth factor
- UA:
-
Uncontrolled asthma
References
Masoli M, Fabian D, Holt S et al (2004) The global burden of asthma: executive summary of the GINA dissemination committee report. Allergy 59(5):469–478. https://doi.org/10.1111/j.1398-9995.2004.00526.x
Stern J, Pier J, Litonjua AA (2020) Asthma epidemiology and risk factors. Semin Immunopathol 42(1):5–15. https://doi.org/10.1007/s00281-020-00785-1
Global Initiative for Asthma (2020) Global Strategy for Asthma Management and Prevention www.ginasthma.org
Hallstrand TS, Leuppi JD, Joos G et al (2018) ERS technical standard on bronchial challenge testing: pathophysiology and methodology of indirect airway challenge testing. Eur Respir J 52(5):1801033. https://doi.org/10.1183/13993003.01033-2018
Kistemaker LE, Gosens R (2015) Acetylcholine beyond bronchoconstriction: roles in inflammation and remodeling. Trends Pharmacol Sci 36(3):164–171. https://doi.org/10.1016/j.tips.2014.11.005
Gori S, Vermeulen M, Remes-Lenicov F et al (2017) Acetylcholine polarizes dendritic cells toward a Th2-promoting profile. Allergy 72(2):221–231. https://doi.org/10.1111/all.12926
Dunford PJ, Holgate ST (2010) The role of histamine in asthma. Adv Exp Med Biol 709:53–66. https://doi.org/10.1007/978-1-4419-8056-4_6
van den Berge M, Polosa R, Kerstjens HA et al (2004) The role of endogenous and exogenous AMP in asthma and chronic obstructive pulmonary disease. J Allergy Clin Immunol 114(4):737–746. https://doi.org/10.1016/j.jaci.2004.05.071
Taylor DA, Jensen MW, Kanabar V et al (1999) A dose-dependent effect of the novel inhaled corticosteroid ciclesonide on airway responsiveness to adenosine-5′-monophosphate in asthmatic patients. Am J Respir Crit Care Med 160(1):237–243. https://doi.org/10.1164/ajrccm.160.1.9809046
Cockcroft DW (2010) Direct challenge tests: airway hyperresponsiveness in asthma: its measurement and clinical significance. Chest 138(2 Suppl):18s–24s. https://doi.org/10.1378/chest.10-0088
Tsurikisawa N, Oshikata C, Tsuburai T et al (2010) Bronchial reactivity to histamine is correlated with airway remodeling in adults with moderate to severe asthma. J Asthma 47(8):841–848. https://doi.org/10.3109/02770903.2010.504876
Kim CK, Hagan JB (2004) Sputum tests in the diagnosis and monitoring of asthma. Ann Allergy Asthma Immunol 93(2):112–122. https://doi.org/10.1016/s1081-1206(10)61462-7
Nathan RA, Sorkness CA, Kosinski M et al (2004) Development of the asthma control test: a survey for assessing asthma control. J Allergy Clin Immunol 113(1):59–65. https://doi.org/10.1016/j.jaci.2003.09.008
Juniper EF, Norman GR, Cox FM et al (2001) Comparison of the standard gamble, rating scale, AQLQ and SF-36 for measuring quality of life in asthma. Eur Respir J 18(1):38–44. https://doi.org/10.1183/09031936.01.00088301
Wang G, Wang F, Gibson PG et al (2017) Severe and uncontrolled asthma in China: a cross-sectional survey from the Australasian severe asthma network. J Thorac Dis 9(5):1333–1344. https://doi.org/10.21037/jtd.2017.04.74
Zhou X, Ding FM, Lin JT et al (2009) Validity of asthma control test for asthma control assessment in Chinese primary care settings. Chest 135(4):904–910. https://doi.org/10.1378/chest.08-0967
Jia CE, Zhang HP, Lv Y et al (2013) The asthma control Test and asthma control questionnaire for assessing asthma control: systematic review and meta-analysis. J Allergy Clin Immunol 131(3):695–703. https://doi.org/10.1016/j.jaci.2012.08.023
Reddel HK, Taylor DR, Bateman ED et al (2009) An official american thoracic society/european respiratory society statement: asthma control and exacerbations: standardizing endpoints for clinical asthma trials and clinical practice. Am J Respir Crit Care Med 180(1):59–99. https://doi.org/10.1164/rccm.200801-060ST
Wang G, Baines KJ, Fu JJ et al (2016) Sputum mast cell subtypes relate to eosinophilia and corticosteroid response in asthma. Eur Respir J 47(4):1123–1133. https://doi.org/10.1183/13993003.01098-2015
Dunn WB, Broadhurst D, Begley P et al (2011) Procedures for large-scale metabolic profiling of serum and serum using gas chromatography and liquid chromatography coupled to mass spectrometry. Nat Protoc 6(7):1060–1083
Wang J, Zhang T, Shen X et al (2016) Serum metabolomics for early diagnosis of esophageal squamous cell carcinoma by UHPLC-QTOF/MS. Metabolomics 12(7):116
Dougherty RH, Fahy JV (2009) Acute exacerbations of asthma: epidemiology, biology and the exacerbation-prone phenotype. Clin Exp Allergy 39(2):193–202. https://doi.org/10.1111/j.1365-2222.2008.03157.x
Park GM, Han HW, Kim JY et al (2016) Association of symptom control with changes in lung function, bronchial hyperresponsiveness, and exhaled nitric oxide after inhaled corticosteroid treatment in children with asthma. Allergol Int 65(4):439–443. https://doi.org/10.1016/j.alit.2016.03.011
Ammar M, Bahloul N, Amri O et al (2022) Oxidative stress in patients with asthma and its relation to uncontrolled asthma. J Clin Lab Anal 36(5):e24345. https://doi.org/10.1002/jcla.24345
Zhang Y, Do DC, Hu X et al (2021) CaMKII oxidation regulates cockroach allergen-induced mitophagy in asthma. J Allergy Clin Immunol 147(4):1464-1477.e11. https://doi.org/10.1016/j.jaci.2020.08.033
Hinchy EC, Gruszczyk AV, Willows R et al (2018) Mitochondria-derived ROS activate AMP-activated protein kinase (AMPK) indirectly. J Biol Chem 293(44):17208–17217. https://doi.org/10.1074/jbc.RA118.002579
Loo SL, Wark PAB (2016) Recent advances in understanding and managing asthma. F1000Res. https://doi.org/10.12688/f1000research.9236.1
Ilmarinen P, Tuomisto LE, Niemelä O et al (2016) Comorbidities and elevated IL-6 associate with negative outcome in adult-onset asthma. Eur Respir J 48(4):1052–1062. https://doi.org/10.1183/13993003.02198-2015
Tanaka T, Narazaki M, Kishimoto T (2014) IL-6 in inflammation, immunity, and disease. Cold Spring Harb Perspect Biol 6(10):a016295. https://doi.org/10.1101/cshperspect.a016295
Panther E, Dürk T, Ferrari D et al (2012) AMP affects intracellular Ca2+ signaling, migration, cytokine secretion and T cell priming capacity of dendritic cells. PLoS ONE 7(5):e37560. https://doi.org/10.1371/journal.pone.0037560
Jakwerth CA, Ordovas-Montanes J, Blank S et al (2022) Role of respiratory epithelial cells in allergic diseases. Cells 11(9):1387
Musiol S, Alessandrini F, Jakwerth CA et al (2022) TGF-β1 drives inflammatory Th cell but not Treg Cell compartment upon allergen exposure. Front Immunol. https://doi.org/10.3389/fimmu.2021.763243
Weckmann M, Bahmer T, Sand JM et al (2021) COL4A3 is degraded in allergic asthma and degradation predicts response to anti-IgE therapy. Eur Respir J 58:200396. https://doi.org/10.1183/13993003.03969-2020
Zissler UM, Jakwerth CA, Guerth F et al (2021) Allergen-specific immunotherapy induces the suppressive secretoglobin 1A1 in cells of the lower airways. Allergy 76(8):2461–2474. https://doi.org/10.1111/all.14756
Liu MC, Bleecker ER, Lichtenstein LM et al (1990) Evidence for elevated levels of histamine, prostaglandin D2, and other bronchoconstricting prostaglandins in the airways of subjects with mild asthma. Am Rev Respir Dis 142(1):126–132. https://doi.org/10.1164/ajrccm/142.1.126
Esther CR Jr, Boysen G et al (2009) Mass spectrometric analysis of biomarkers and dilution markers in exhaled breath condensate reveals elevated purines in asthma and cystic fibrosis. Am J Physiol Lung Cell Mol Physiol 296(6):L987–L993. https://doi.org/10.1152/ajplung.90512.2008
Yamauchi K, Ogasawara M (2019) The Role of histamine in the pathophysiology of asthma and the clinical efficacy of antihistamines in asthma therapy. Int J Mol Sci. https://doi.org/10.3390/ijms20071733
Koarai A, Ichinose M, Ishigaki-Suzuki S et al (2003) Disruption of L-histidine decarboxylase reduces airway eosinophilia but not hyperresponsiveness. Am J Respir Crit Care Med 167(5):758–763. https://doi.org/10.1164/rccm.200206-619OC
Matsuda T, Suzuki Y, Fujisawa T et al (2020) Imaging mass spectrometry to visualise increased acetylcholine in lungs of asthma model mice. Anal Bioanal Chem 412(18):4327–4341. https://doi.org/10.1007/s00216-020-02670-0
Gosens R, Zaagsma J, Meurs H et al (2006) Muscarinic receptor signaling in the pathophysiology of asthma and COPD. Respir Res 7(1):73. https://doi.org/10.1186/1465-9921-7-73
Pieper MP (2012) The non-neuronal cholinergic system as novel drug target in the airways. Life Sci 91(21–22):1113–1118. https://doi.org/10.1016/j.lfs.2012.08.030
Acknowledgements
The authors are grateful to Ms. Michelle Gleeson (Hunter Medical Research Institute, the University of Newcastle, Australia), Ms. Zhi Lin (West China Hospital, Sichuan University, China) for their sputum processing. The authors also thank all the participants for participating in this study.
Funding
This study was supported by the National Natural Science Foundation of China (Grant Nos. 81920108002, 81870027, and 81900026), and 1.3.5 project for disciplines of excellence-Clinical Research Incubation Project, West China Hospital, Sichuan University (Grant No. 2018HXFH016), and Post-Doctor Research Project, West China Hospital, Sichuan University, China (Grant No. 2021HXBH013).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by all authors. The first draft of the manuscript was written by XMF, YL, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
G. Wang reports personal fees from AstraZeneca, GlaxoSmithKline, Novartis, Chiesi; and grants from AstraZeneca outside the submitted work. The rest of the authors declare that they have no relevant conflicts of interest.
Ethical Approval
This study was approved by the Institutional Review Board of West China Hospital, Sichuan University (No. 2014–30).
Consent to Participate
Written informed consent was sought and obtained from all participants before enrollment.
Consent to Publications
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fang, X.M., Liu, Y., Wang, J. et al. Endogenous Adenosine 5′-Monophosphate, But Not Acetylcholine or Histamine, is Associated with Asthma Control, Quality of Life, and Exacerbations. Lung 200, 579–589 (2022). https://doi.org/10.1007/s00408-022-00570-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00408-022-00570-x