Abstract
Purpose
Considering the current recommendation of the World Health Organization to replace sputum smear microscopy with Xpert MTB/RIF as an initial diagnostic test for tuberculosis (TB), and that culture takes time to provide results, the cycle threshold (CT) of the Xpert test may be the only way to assess bacillary load. The objective of this study is to evaluate the association of bacillary load, measured by the Xpert CT, with the TB treatment outcomes.
Methods
In cohort study, Xpert CT values were evaluated in cured and non-cured (failure and death) patients. Multivariate analysis was performed to evaluate if CT is independently associated with TB treatment outcomes.
Results
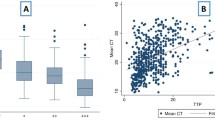
During this study period, 155 patients (84 cured and 71 non-cured) met the inclusion and were included in the analysis. The mean CT value for Xpert MTB/RIF test was 20.7 ± 5.6 in cured patients and 17.1 ± 5.6 in non-cured patients (p < 0.0001). Previous TB was more frequent in non-cured (28.2%) than in cured patients (7.1%) (p < 0.0001). Non-cured patients were younger than cured ones (37.1 ± 13.3 vs 43.6 ± 16.2; p = 0.006). HIV was more frequent in non-cured (28.2%) than in cured patients (15.5%), although this difference was not statistically significant (p = 0.054). In multivariate analysis, CT values, age, previous TB, and HIV were independently associated with non-cure.
Conclusions
Lower Xpert MTB/RIF CT values were independently associated with worse treatment outcomes. The information from even a single test performed before starting treatment proved to be a relatively good predictor of TB treatment outcome.
Similar content being viewed by others
Data Availability
Data are available at Mendeley.
References
World Health Organization (2019) Global tuberculosis report. www.who.int
Boehme CC, Nabeta P, Hillemann D et al (2010) Rapid molecular detection of tuberculosis and rifampin resistance. N Engl J Med 363:1005–1020. https://doi.org/10.1056/NEJMoa0907847
Anvisa Boletim Brasileiro de Avaliação de Tecnologias em Saúde (BRATS) no 16 - Busca - Anvisa. https://portal.anvisa.gov.br/resultado-de-busca?p_p_id=101&p_p_lifecycle=0&p_p_state=maximized&p_p_mode=view&p_p_col_id=column-1&p_p_col_count=1&_101_struts_action=%2Fasset_publisher%2Fview_content&_101_assetEntryId=412399&_101_type=document. Accessed 2 June 2020
World Health Organization (2011) Automated Real-time Nucleic Acid Amplification Technology for Rapid and Simultaneous Detection of Tuberculosis and Rifampicin Resistance: Xpert MTB/RIF System Policy Statement WHO Library Cataloguing-in-Publication Data Policy statement: automated real-ti
Lange B, Khan P, Kalmambetova G et al (2017) Diagnostic accuracy of the Xpert® MTB/RIF cycle threshold level to predict smear positivity: a meta-analysis. Int J Tuberc Lung Dis 21:493–502. https://doi.org/10.5588/ijtld.16.0702
Beynon F, Theron G, Respeito D et al (2018) Correlation of Xpert MTB/RIF with measures to assess Mycobacterium tuberculosis bacillary burden in high HIV burden areas of Southern Africa. Sci Rep 8:1–9. https://doi.org/10.1038/s41598-018-23066-2
Hanrahan CF, Theron G, Bassett J et al (2014) Xpert MTB/RIF as a measure of sputum bacillary burden: variation by HIV status and immunosuppression. Am J Respir Crit Care Med 189:1426–1434. https://doi.org/10.1164/rccm.201312-2140OC
Secretaria de Vigilância em Saúde | Ministério da (2020) Boletim Epidemiológico - Tuberculose 2020. https://www.saude.gov.br/images/pdf/2020/marco/24/Boletim-tuberculose-2020-marcas--1-.pdf
Conde MB, de Melo FAF, Marques AMC et al (2009) III Brazilian Thoracic Association Guidelines on tuberculosis. J Bras Pneumol 35:1018–1048
American Thoracic Society (2000) Diagnostic standards and classification of tuberculosis in adults and children. Am J Respir Crit Care Med 161:1376–1395. https://doi.org/10.1164/ajrccm.161.4.16141
Perrin FMR, Woodward N, Phillips PPJ et al (2010) Radiological cavitation, sputum mycobacterial load and treatment response in pulmonary tuberculosis. Int J Tuberc Lung Dis 14:1596–1602
Pheiffer C, Carroll NM, Beyers N et al (2008) Time to detection of Mycobacterium tuberculosis in BACTEC systems as a viable alternative to colony counting. Int J Tuberc Lung Dis 12:792–798
Diacon AH, Maritz JS, Venter A et al (2010) Time to detection of the growth of Mycobacterium tuberculosis in MGIT 960 for determining the early bactericidal activity of antituberculosis agents. Eur J Clin Microbiol Infect Dis 29:1561–1565. https://doi.org/10.1007/s10096-010-1043-7
Palaci M, Dietze R, Hadad DJ et al (2007) Cavitary disease and quantitative sputum bacillary load in cases of pulmonary tuberculosis we examined sputum bacterial loads in adults with newly diagnosed tuberculosis using quantitative culture and time-until-positive (DTP) culture in BACTEC 460. Patients with cavitary disease had higher CFU levels than those without cavities and shorter DTPs. Within radiographic strata of moderately and far advanced tuberculosis, higher CFU counts were associated with cavitary disease. J Clin Microbiol 45:4064–4066. https://doi.org/10.1128/JCM.01780-07
Shenai S, Ronacher K, Malherbe S et al (2016) Bacterial loads measured by the xpert MTB/ RIF assay as markers of culture conversion and bacteriological cure in pulmonary TB. PLoS ONE 11:1–13. https://doi.org/10.1371/journal.pone.0160062
Tweya H, Feldacker C, Phiri S et al (2013) Comparison of treatment outcomes of new smear-positive pulmonary tuberculosis patients by HIV and antiretroviral status in a TB/HIV clinic, Malawi. PLoS ONE. https://doi.org/10.1371/journal.pone.0056248
Mabunda TE, Ramalivhana NJ, Dambisya YM (2014) Mortality associated with tuberculosis/HIV co-infection among patients on TB treat-ment in the Limpopo province South Africa . Afr Health Sci. https://doi.org/10.4314/ahs.v14i4.12
Ambadekar NN, Zodpey SP, Soni RN, Lanjewar SP (2015) Treatment outcome and its attributes in TB-HIV co-infected patients registered under Revised National TB Control Program: a retrospective cohort analysis. Public Health 129:783–789. https://doi.org/10.1016/j.puhe.2015.03.006
Lee J, Woo Nam H, Ha Choi S et al (2017) Comparison of early and late tuberculosis deaths in Korea. J Korean Med Sci 32:700–703. https://doi.org/10.3346/jkms.2017.32.4.700
Lefebvre N, Falzon D, Falzon CD (2008) Risk factors for death among tuberculosis cases: analysis of European surveillance data. Eur Respir J Eur Respir J 31:1256–1260. https://doi.org/10.1183/09031936.00131107
Shuldiner J, Leventhal A, Chemtob D, Mor Z (2014) Mortality of tuberculosis patients during treatment in Israel, 2000–2010. Int J Tuberc Lung Dis. https://doi.org/10.5588/ijtld.13.0591
Kolappan C, Subramani R, Karunakaran K, Narayanan PR (2006) Mortality of tuberculosis patients in Chennai, India. Bull World Health Organ 84:555–560. https://doi.org/10.2471/BLT.05.022087
Sarker M, Homayra F, Rawal LB et al (2019) Urban-rural and sex differentials in tuberculosis mortality in Bangladesh: results from a population-based survey. Trop Med Int Heal 24:109–115. https://doi.org/10.1111/tmi.13171
Roth GA, Abate D, Abate KH et al (2018) Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 392:1736–1788. https://doi.org/10.1016/S0140-6736(18)32203-7
Alavi-Naini R, Moghtaderi A, Metanat M et al (2013) Factors associated with mortality in tuberculosis patients. J Res Med Sci 18:52–55
Osman M, Welte A, Dunbar R et al (2019) Morbidity and mortality up to 5 years post tuberculosis treatment in South Africa: a pilot study. Int J Infect Dis 85:57–63. https://doi.org/10.1016/j.ijid.2019.05.024
El-Shabrawy M, El-Shafei DA (2017) Evaluation of treatment failure outcome and its predictors among pulmonary tuberculosis patients in Sharkia Governorate, 2013–2014. Egypt J Chest Dis Tuberc 66:145–152. https://doi.org/10.1016/j.ejcdt.2015.11.002
Funding
Fundo de Incentivo à Pesquisa do Hospital de Clínicas de Porto Alegre (FIPE-HCPA).
Author information
Authors and Affiliations
Contributions
MMP designed the work, collected and analyzed data, and wrote the manuscript. GRP, MSB, NJDS, CS, and JSH designed the work, analyzed data, revised the manuscript. DRS designed the work, analyzed data, and wrote the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to declare.
Ethical Approval
The study was approved by the Research Ethics Committee of Hospital de Clínicas de Porto Alegre (number 160063), and all research was in accordance with regulations.
Consent to Participate
All cases and controls signed informed consent form prior to inclusion in the study.
Consent for Publication
All authors approved the submitted version.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pires, M.d., Pereira, G.R., Barbosa, M.S. et al. Association of Xpert MTB/RIF Cycle Threshold Values with Tuberculosis Treatment Outcomes. Lung 198, 985–989 (2020). https://doi.org/10.1007/s00408-020-00398-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00408-020-00398-3