Abstract
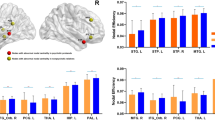
Negative symptoms, including avolition, anhedonia, asociality, blunted affect and alogia are associated with poor long-term outcome and functioning. However, treatment options for negative symptoms are limited and neurobiological mechanisms underlying negative symptoms in schizophrenia are still poorly understood. Diffusion-weighted magnetic resonance imaging scans were acquired from 64 patients diagnosed with schizophrenia and 35 controls. Global and regional network properties and rich club organization were investigated using graph analytical methods. We found that the schizophrenia group had higher modularity, clustering coefficient and characteristic path length, and lower rich connections compared to controls, suggesting highly connected nodes within modules but less integrated with nodes in other modules in schizophrenia. We also found a lower nodal degree in the left thalamus and left putamen in schizophrenia relative to the control group. Importantly, higher modularity was associated with greater negative symptoms but not with cognitive deficits in patients diagnosed with schizophrenia suggesting an alteration in modularity might be specific to overall negative symptoms. The nodal degree of the left thalamus was associated with both negative and cognitive symptoms. Our findings are important for improving our understanding of abnormal white-matter network topology underlying negative symptoms in schizophrenia.



Similar content being viewed by others
Availability of data and materials
All data and materials are available on request.
Code availability
Not applicable.
References
Javitt DC (2001) Management of negative symptoms of schizophrenia. Curr Psychiatry Rep 3:413–417
Kirkpatrick B, Buchanan RW, Ross DE, Carpenter WT Jr (2001) A separate disease within the syndrome of schizophrenia. Arch Gen Psychiatry 58:165–171
Galderisi S, Bucci P, Mucci A et al (2013) Categorical and dimensional approaches to negative symptoms of schizophrenia: focus on long-term stability and functional outcome. Schizophr Res 147:157–162
Rabinowitz J, Levine SZ, Garibaldi G, Bugarski-Kirola D, Berardo CG, Kapur S (2012) Negative symptoms have greater impact on functioning than positive symptoms in schizophrenia: analysis of CATIE data. Schizophr Res 137:147–150
Robertson BR, Prestia D, Twamley EW, Patterson TL, Bowie CR, Harvey PD (2014) Social competence versus negative symptoms as predictors of real world social functioning in schizophrenia. Schizophr Res 160:136–141
Begue I, Kaiser S, Kirschner M (2020) Pathophysiology of negative symptom dimensions of schizophrenia - Current developments and implications for treatment. Neurosci Biobehav Rev 116:74–88
Fusar-Poli P, Papanastasiou E, Stahl D et al (2015) Treatments of negative symptoms in schizophrenia: meta-analysis of 168 randomized placebo-controlled trials. Schizophr Bull 41:892–899
Uranova NA, Vostrikov VM, Orlovskaya DD, Rachmanova VI (2004) Oligodendroglial density in the prefrontal cortex in schizophrenia and mood disorders: a study from the Stanley Neuropathology Consortium. Schizophr Res 67:269–275
Uranova N, Orlovskaya D, Vikhreva O et al (2001) Electron microscopy of oligodendroglia in severe mental illness. Brain Res Bull 55:597–610
Kelly S, Jahanshad N, Zalesky A et al (2018) Widespread white matter microstructural differences in schizophrenia across 4322 individuals: results from the ENIGMA Schizophrenia DTI Working Group. Mol Psychiatry 23:1261–1269
Klauser P, Baker ST, Cropley VL et al (2017) White matter disruptions in schizophrenia are spatially widespread and topologically converge on brain network hubs. Schizophr Bull 43:425–435
Yang X, Cao D, Liang X, Zhao J (2017) Schizophrenia symptomatic associations with diffusion tensor imaging measured fractional anisotropy of brain: a meta-analysis. Neuroradiology 59:699–708
Huang JY, Liu CM, Hwang TJ et al (2018) Shared and distinct alterations of white matter tracts in remitted and nonremitted patients with schizophrenia. Hum Brain Mapp 39:2007–2019
Ochi R, Noda Y, Tsuchimoto S et al (2020) White matter microstructural organizations in patients with severe treatment-resistant schizophrenia: A diffusion tensor imaging study. Prog Neuropsychopharmacol Biol Psychiatry 100:109871
Zeng B, Ardekani BA, Tang Y et al (2016) Abnormal white matter microstructure in drug-naive first episode schizophrenia patients before and after eight weeks of antipsychotic treatment. Schizophr Res 172:1–8
van den Heuvel MP, Fornito A (2014) Brain networks in schizophrenia. Neuropsychol Rev 24:32–48
Zhang X, Braun U, Harneit A et al (2021) Generative network models of altered structural brain connectivity in schizophrenia. Neuroimage 225:117510
Stephan KE, Friston KJ, Frith CD (2009) Dysconnection in schizophrenia: from abnormal synaptic plasticity to failures of self-monitoring. Schizophr Bull 35:509–527
Bullmore E, Sporns O (2009) Complex brain networks: graph theoretical analysis of structural and functional systems. Nat Rev Neurosci 10:186–198
Lord LD, Stevner AB, Deco G, Kringelbach ML (2017) Understanding principles of integration and segregation using whole-brain computational connectomics: implications for neuropsychiatric disorders. Philos Trans A Math Phys Eng Sci. https://doi.org/10.1098/rsta.2016.0283
van den Heuvel MP, Sporns O, Collin G et al (2013) Abnormal rich club organization and functional brain dynamics in schizophrenia. JAMA Psychiat 70:783–792
Zalesky A, Fornito A, Seal ML et al (2011) Disrupted axonal fiber connectivity in schizophrenia. Biol Psychiatry 69:80–89
Schmidt A, Crossley NA, Harrisberger F et al (2017) Structural network disorganization in subjects at clinical high risk for psychosis. Schizophr Bull 43:583–591
Wang S, Gong G, Zhong S et al (2020) Neurobiological commonalities and distinctions among 3 major psychiatric disorders: a graph theoretical analysis of the structural connectome. J Psychiatry Neurosci 45:15–22
Shon SH, Yoon W, Kim H, Joo SW, Kim Y, Lee J (2018) Deterioration in global organization of structural brain networks in schizophrenia: a diffusion MRI tractography study. Front Psychiatry 9:272
Cea-Canas B, de Luis R, Lubeiro A et al (2019) Structural connectivity in schizophrenia and bipolar disorder: Effects of chronicity and antipsychotic treatment. Prog Neuropsychopharmacol Biol Psychiatry 92:369–377
Sun Y, Chen Y, Lee R, Bezerianos A, Collinson SL, Sim K (2016) Disruption of brain anatomical networks in schizophrenia: A longitudinal, diffusion tensor imaging based study. Schizophr Res 171:149–157
van den Heuvel MP, Sporns O (2011) Rich-club organization of the human connectome. J Neurosci 31:15775–15786
de Lange SC, Scholtens LH, Initiative ADN et al (2019) Shared vulnerability for connectome alterations across psychiatric and neurological brain disorders. Nat Hum Behav 3:988–998
Cui LB, Wei Y, Xi YB et al (2019) Connectome-based patterns of first-episode medication-naive patients with schizophrenia. Schizophr Bull 45:1291–1299
Collin G, Kahn RS, de Reus MA, Cahn W, van den Heuvel MP (2014) Impaired rich club connectivity in unaffected siblings of schizophrenia patients. Schizophr Bull 40:438–448
Michielse S, Rakijo K, Peeters S et al (2019) Microstructural white matter network-connectivity in individuals with psychotic disorder, unaffected siblings and controls. Neuroimage Clin 23:101931
Wei Q, Zhao L, Zou Y et al (2020) The role of altered brain structural connectivity in resilience, vulnerability, and disease expression to schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry 101:109917
Alloza C, Bastin ME, Cox SR et al (2017) Central and non-central networks, cognition, clinical symptoms, and polygenic risk scores in schizophrenia. Hum Brain Mapp 38:5919–5930
Zhang R, Wei Q, Kang Z et al (2015) Disrupted brain anatomical connectivity in medication-naive patients with first-episode schizophrenia. Brain Struct Funct 220:1145–1159
Wang Q, Su TP, Zhou Y et al (2012) Anatomical insights into disrupted small-world networks in schizophrenia. Neuroimage 59:1085–1093
Sporns O, Betzel RF (2016) Modular brain networks. Annu Rev Psychol 67:613–640
Lerman-Sinkoff DB, Barch DM (2016) Network community structure alterations in adult schizophrenia: identification and localization of alterations. Neuroimage Clin 10:96–106
Scariati E, Schaer M, Karahanoglu I et al (2016) Large-scale functional network reorganization in 22q11.2 deletion syndrome revealed by modularity analysis. Cortex 82:86–99
Collin G, Seidman LJ, Keshavan MS et al (2020) Functional connectome organization predicts conversion to psychosis in clinical high-risk youth from the SHARP program. Mol Psychiatry 25:2431–2440
Andreasen NC (1984): Scale for the assessment of positive symptoms. Iowa City: University of Iowa
Kirkpatrick B, Strauss GP, Nguyen L et al (2011) The brief negative symptom scale: psychometric properties. Schizophr Bull 37:300–305
PolatNazli I, Ergul C, Aydemir O, Chandhoke S, Ucok A, Gonul AS (2016) Validation of Turkish version of brief negative symptom scale. Int J Psychiatry Clin Pract 20:265–271
Addington D, Addington J, Maticka-Tyndale E (1994) Specificity of the Calgary Depression Scale for schizophrenics. Schizophr Res 11:239–244
Aydemir Ö, EsenDanacı A, Deveci A, İçelli İ (2000) Calgary şizofrenide depresyon ölçeği’nin Türkçe versiyonunun güvenilirliği ve geçerliliği. Nöropsikiyatri Arşivi 37:82–86
Tournier JD, Smith R, Raffelt D et al (2019) MRtrix3: A fast, flexible and open software framework for medical image processing and visualisation. Neuroimage 202:116137
Dikmeer N, Besiroglu L, Di Biase MA, Zalesky A, Kasal MI, Bilge A et al (2021) White matter microstructure and connectivity in patients with obsessive-compulsive disorder and their unaffected siblings. Acta Psychiatr Scand. 143:72–81
Veraart J, Novikov DS, Christiaens D, Ades-Aron B, Sijbers J, Fieremans E (2016) Denoising of diffusion MRI using random matrix theory. Neuroimage 142:394–406
Kellner E, Dhital B, Kiselev VG, Reisert M (2016) Gibbs-ringing artifact removal based on local subvoxel-shifts. Magn Reson Med 76:1574–1581
Andersson JL, Sotiropoulos SN (2016) An integrated approach to correction for off-resonance effects and subject movement in diffusion MR imaging. Neuroimage 125:1063–1078
Tustison NJ, Avants BB, Cook PA et al (2010) N4ITK: improved N3 bias correction. IEEE Trans Med Imaging 29:1310–1320
Tournier JD, Calamante F, Connelly A (2013) Determination of the appropriate b value and number of gradient directions for high-angular-resolution diffusion-weighted imaging. NMR Biomed 26:1775–1786
Tournier JD, Calamante F, Connelly A (2007) Robust determination of the fibre orientation distribution in diffusion MRI: non-negativity constrained super-resolved spherical deconvolution. Neuroimage 35:1459–1472
Smith RE, Tournier JD, Calamante F, Connelly A (2012) Anatomically-constrained tractography: improved diffusion MRI streamlines tractography through effective use of anatomical information. Neuroimage 62:1924–1938
Smith RE, Tournier J-D, Calamante F, Connelly A (2013) SIFT: Spherical-deconvolution informed filtering of tractograms. Neuroimage 67:298–312
Tzourio-Mazoyer N, Landeau B, Papathanassiou D et al (2002) Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 15:273–289
Hagmann P, Cammoun L, Gigandet X et al (2008) Mapping the structural core of human cerebral cortex. PLoS Biol 6:e159
de Reus MA, van den Heuvel MP (2013) Estimating false positives and negatives in brain networks. Neuroimage 70:402–409
Reess TJ, Rus OG, Schmidt R et al (2016) Connectomics-based structural network alterations in obsessive-compulsive disorder. Transl Psychiatry 6:e882
van Wijk BC, Stam CJ, Daffertshofer A (2010) Comparing brain networks of different size and connectivity density using graph theory. PLoS ONE 5:e13701
Zorlu N, Capraz N, Oztekin E et al (2019) Rich club and reward network connectivity as endophenotypes for alcohol dependence: a diffusion tensor imaging study. Addict Biol 24:265–274
Rubinov M, Sporns O (2010) Complex network measures of brain connectivity: uses and interpretations. Neuroimage 52:1059–1069
Newman ME (2006) Modularity and community structure in networks. Proc Natl Acad Sci U S A 103:8577–8582
Bullmore E, Sporns O (2012) The economy of brain network organization. Nat Rev Neurosci 13:336–349
Maslov S, Sneppen K (2002) Specificity and stability in topology of protein networks. Science 296:910–913
Kim S (2015) ppcor: an R package for a fast calculation to semi-partial correlation coefficients. Commun Stat Appl Methods 22:665–674
Li F, Lui S, Yao L et al (2018) Altered white matter connectivity within and between networks in antipsychotic-naive first-episode schizophrenia. Schizophr Bull 44:409–418
Yeo RA, Ryman SG, van den Heuvel MP et al (2016) Graph metrics of structural brain networks in individuals with schizophrenia and healthy controls: group differences, relationships with intelligence, and genetics. J Int Neuropsychol Soc 22:240–249
Zalesky A, Fornito A, Harding IH et al (2010) Whole-brain anatomical networks: does the choice of nodes matter? Neuroimage 50:970–983
Wang H, Zeng LL, Chen Y, Yin H, Tan Q, Hu D (2015) Evidence of a dissociation pattern in default mode subnetwork functional connectivity in schizophrenia. Sci Rep 5:14655
Brady RO Jr, Gonsalvez I, Lee I et al (2019) Cerebellar-prefrontal network connectivity and negative symptoms in schizophrenia. Am J Psychiatry 176:512–520
Manoliu A, Riedl V, Doll A et al (2013) Insular dysfunction reflects altered between-network connectivity and severity of negative symptoms in schizophrenia during psychotic remission. Front Hum Neurosci 7:216
Andreasen NC (1997) The role of the thalamus in schizophrenia. Can J Psychiatry 42:27–33
Domen P, Peeters S, Michielse S et al (2017) Differential time course of microstructural white matter in patients with psychotic disorder and individuals at risk: a 3-year follow-up study. Schizophr Bull 43:160–170
Viher PV, Stegmayer K, Giezendanner S et al (2016) Cerebral white matter structure is associated with DSM-5 schizophrenia symptom dimensions. Neuroimage Clin 12:93–99
Melicher T, Horacek J, Hlinka J et al (2015) White matter changes in first episode psychosis and their relation to the size of sample studied: a DTI study. Schizophr Res 162:22–28
Bopp MHA, Zollner R, Jansen A, Dietsche B, Krug A, Kircher TTJ (2017) White matter integrity and symptom dimensions of schizophrenia: A diffusion tensor imaging study. Schizophr Res 184:59–68
Cheng W, Palaniyappan L, Li M et al (2015) Voxel-based, brain-wide association study of aberrant functional connectivity in schizophrenia implicates thalamocortical circuitry. NPJ Schizophr 1:15016
Chen P, Ye E, Jin X, Zhu Y, Wang L (2019) Association between thalamocortical functional connectivity abnormalities and cognitive deficits in schizophrenia. Sci Rep 9:2952
Martino M, Magioncalda P, Yu H et al (2018) Abnormal resting-state connectivity in a substantia nigra-related striato-thalamo-cortical network in a large sample of first-episode drug-naive patients with schizophrenia. Schizophr Bull 44:419–431
Avram M, Brandl F, Bauml J, Sorg C (2018) Cortico-thalamic hypo- and hyperconnectivity extend consistently to basal ganglia in schizophrenia. Neuropsychopharmacology 43:2239–2248
Giraldo-Chica M, Rogers BP, Damon SM, Landman BA, Woodward ND (2018) Prefrontal-thalamic anatomical connectivity and executive cognitive function in schizophrenia. Biol Psychiatry 83:509–517
Jbabdi S, Johansen-Berg H (2011) Tractography: where do we go from here? Brain Connect 1:169–183
Jones DK, Knosche TR, Turner R (2013) White matter integrity, fiber count, and other fallacies: the do’s and don’ts of diffusion MRI. Neuroimage 73:239–254
Calamuneri A, Arrigo A, Mormina E et al (2018) White matter tissue quantification at low b-values within constrained spherical deconvolution framework. Front Neurol 9:716
Funding
This work was supported by Research Fund of Izmir Katip Celebi University (Project number: 2018-GAP-TIPF-0003) which had no role in the design of the study, collection and analysis of data and decision to publish.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Consent to participate
All authors agreed to participate the study.
Consent for publication
All authors agreed with publication of this study.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bayrakçı, A., Zorlu, N., Karakılıç, M. et al. Negative symptoms are associated with modularity and thalamic connectivity in schizophrenia. Eur Arch Psychiatry Clin Neurosci 273, 565–574 (2023). https://doi.org/10.1007/s00406-022-01433-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00406-022-01433-5