Abstract
Insight into illness in schizophrenia (SZ) patients has a major impact on treatment adherence and outcome. Previous studies have linked distinct deviations of brain structure to illness insight, specifically in frontoparietal and subcortical regions. Some of these abnormalities are thought to reflect aberrant cortical development. In this study, we used cross-sectional data to examine associations between illness insight and two cortical surface markers that are known to follow distinct neurodevelopmental trajectories, i.e. cortical gyrification (CG) and thickness (CT). CG and CT was investigated in SZ patients (n = 82) and healthy controls (HC, n = 48) using 3 T structural magnetic resonance imaging. Illness insight in SZ patients was measured using the OSSTI scale, an instrument that provides information on two distinct dimensions of illness insight, i.e. treatment adherence (OSSTI-A) and identification of disease-related symptoms (OSSTI-I). CT and CG were computed using the Computational Anatomy Toolbox (CAT12). Whole-brain and regions-of-interest (ROI)-based analyses were performed. SZ patients showed higher CG in anterior cingulate, superior frontal and temporal gyrus and reduced CG in insular and superior frontal cortex when compared to HC. SZ patients showed decreased CT in pre- and paracentral, occipital, cingulate, frontoparietal and temporal regions. Illness insight in SZ patients was significantly associated with both CG and CT in the left inferior parietal lobule (OSSTI-A) and the right precentral gyrus (CG/OSSTI-A, CT/OSSTI-I). The data support a multi-parametric neuronal model with both pre- and postnatal brain developmental factors having an impact on illness insight in patients with SZ.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Poor illness insight is a common feature of schizophrenia (SZ) and is associated with unfavourable long-term outcomes. Poor illness insight has been related to poor treatment adherence, poor clinical outcome, increased negative and positive symptoms and adverse integration into the community. In contrast, well-preserved illness insight has been linked to better metacognitive and psychosocial function, fewer relapses, decreased number of hospitalizations, and higher quality of life [36]. Previous studies have shown that illness insight is not a stable feature of the disorder but fluctuates over time, as a function of the natural course of the disease or modulated by treatment [40]. Although a plethora of studies so far have investigated illness insight in SZ, this clinically important feature still lacks a consistent, generally accepted definition. In this study, illness insight was defined as the patients’ ability to understand SZ as a disease they are suffering from, to correctly attribute symptoms as disease-related, and to accept the need of treatment and related treatment adherence.
Despite its clinical relevance, little is known about the neurobiological underpinnings of illness insight in SZ. In the last 2 decades, we have witnessed an increased effort to identify neuronal correlates of the different levels of illness insight, with varying results. Previous magnetic resonance imaging (MRI) studies on illness insight used distinct methodological approaches such as voxel- or surface-based morphometry (VBM, SBM) and reported alterations in networks comprising frontal, parietal, temporal, basal ganglia and cerebellar regions, respectively [11, 13, 36]. For instance, Cooke et al. reported positive associations between illness insight and grey matter volume (GMV) in temporal cortex, right parietal gyrus and precuneus [13]. SBM studies examining relationships between illness insight and cortical thickness (CT) reported associations between poor awareness of illness and lower CT in the left middle frontal and inferior temporal gyri, while poor awareness of treatment need and efficacy were found to be related to lower CT in the left medial frontal gyrus, precuneus and temporal gyri [11]. Symptom misattribution was associated with widespread variations in CT in frontal, occipital, temporal and parietal regions [10]. Finally, higher self-reflectiveness was associated with thinner CT in the right occipital cortex [10]. Emami et al. showed lower CT in the right superior temporal gyrus, insula and parahippocampal gyrus in SZ patients with low insight [16]. Yet, other researchers were not able to identify any associations between illness insight and brain structure or function [5, 6]. As an example, Beland et al. could not find any significant relationships between CT and illness insight [6].
To date, however, it is unclear whether poor illness insight in SZ is differentially linked to cortical features of distinct evolutionary and genetic origin such as cortical gyrification (CG) and CT [25, 47]. To fill this gap, this MRI study examined the relationship between inter-individual variations of illness insight and CG and CT in SZ patients. On one side, CG is developed in the early stages of brain development, particularly during weeks 24 and 32 of gestation and undergoes changes until the early postnatal period [4, 19]. On the other side, CT undergoes dynamic development during the first 2 decades of life and is modulated by genetic as well as environmental factors (e.g., drug abuse, trauma, urban upbringing, etc.) [20, 22, 52, 62]. Therefore, analysing CG and CT separately makes it possible to differentiate between early cortical neurodevelopment and other ongoing neurodevelopmental processes during adolescence and young adulthood. Furthermore, the separate examination of cortical measures with temporally different neurodevelopmental origin will help to decipher the different contributions of CG and CT to illness insight in SZ. In this study, we used structural MRI and SBM to investigate relationships between cortical morphology and illness insight in patients with SZ. Illness insight was measured using the German version of the “Osnabrueck Scale of Therapeutic Attitudes and Identification of Psychological Problems in Schizophrenia” (OSSTI) [31, 59], an instrument that allows the assessment of distinct insight dimensions that are also related to treatment adherence and not solely into insight whether a disease is present or not. Assuming a multi-parametric neural model of illness insight in SZ influenced by the prenatal as well as postnatal neurodevelopment, we expected to find significant associations between OSSTI scores and both CG and CT. More precisely, we predicted a significant association between OSSTI scores and both CG and CT alterations in brain regions, especially prefrontal and inferior parietal regions that have been previously linked to self-perception, self-awareness [21, 46, 60], and “anosognosia” [38, 45, 57].
Methods
Participants
In this study, the original sample size consisted of 135 participants. MRI data quality procedures (see below) led to the exclusion of five participants (four SZ, one healthy participant). Eighty-two patients with SZ and 48 healthy controls (HC) were considered in within- and between-group analyses; detailed demographic and clinical data are shown in Table 1. SZ patients were consecutively recruited from the Department of Psychiatry and Psychotherapy at the Central Institute of Mental Health in Mannheim, Germany. Inclusion criteria consisted of a diagnosis of paranoid SZ according to ICD-10 (F20.0) with stable medication for at least 2 weeks, right-handedness, and age between 18 and 65 years. Exclusion criteria were a history of any substance dependency except for tobacco. HC were recruited through advertisements and screened for major psychiatric disorders before being included. Clinical evaluation included ascertainment of personal and family history and detailed physical and neurological examination. None of the HC had a lifetime history of neurological or medical illness, head injury, or substance abuse. All included study participants were right‐handed according to the Edinburgh Handedness Inventory [42]. All procedures contributing to this work complied with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised 2008. The local ethics committee (Medical Faculty at Heidelberg University, Germany) approved the study. Written informed consent was obtained from all participants following a complete description of the study.
Illness insight was measured with the OSSTI by Krupa in 2005 [31, 59]. We chose this scale because it comprises two dimensions of illness insight separately, adherence (OSSTI-A) and identification of disease-related symptoms (OSSTI-I). The advantage of this scale compared to other scales is the separate analysis of adherence (as one of two sub-scales) and identification of disease-related symptoms in SZ. Using this parameter, our study was able to analyse the influence of adherence as a clinically decisive parameter of illness insight, as non-adherence is associated with higher relapse risk and hospitalization [43]. The OSSTI is a self-rated instrument that was developed by Krupa [31, 59] based on the “Scale to Assess Unawareness of Mental Disorder” (SUMD) [1], Birchwood's Insight Scale (BIS) [7] and the “Self-Appraisal of Illness Questionnaire” (SAIQ) [37]. The original version comprised 24 items (6-point Likert scales), which were assigned to three sub-scales (1: identification of psychotic symptoms; 2: need for treatment and compliance; 3: consequences of mental illness). The first discriminatory test was performed in a sample of 26 patients with schizophrenia spectrum disorders. It resulted in the complete exclusion of scale 3 and a further exclusion of six items. Specifically, the OSSTI is a self-report instrument that comprises ten Likert-scale (six levels) items in two factors (OSSTI-A and OSSTI-I; scores by factor were used in this study). As an example of the items in these factors, statements like “I am healthy and have no mental complaints.” or “I tell my friends/my family/my acquaintances about my mental problems to prevent misunderstandings.” are used in the OSSTI. In a study comprising 85 schizophrenia patients, an acceptable Cronbach’s α for standardized items of 0.79 was detected [59]. Krupa reported consistencies of α1 = 0.67 (items 2, 4, 6, 8) and α2 = 0.77 (items 1, 3, 5, 7, 9, 10) for the final version. However, further psychometric analyses could not take place due to the sample size, validity evidence was not provided so far. The study by Waldorf on 85 SZ patients detected a Cronbach’s α for standardized items of 0.79, mean item intercorrelation of 0.28 and significant correlation with PANSS G12 item of r = 0.54/p < 0.001. Further psychometric assessment included the Positive and Negative Syndrome Scale (PANSS) [28].
Structural neuroimaging data acquisition
Structural data were acquired using T1-weigthed three-dimensional (3D) magnetization-prepared rapid gradient-echo at the Central Institute of Mental Health, Mannheim, Germany on a 3.0 Tesla Magnetom Tim Trio MRI scanner (Siemens Medical Systems) with the following parameters: flip angle 7°, echo time (TE) = 3.93 ms; repetition time (TR) = 2530 ms; inversion time (TI) = 1100 ms; FOV = 256 mm; slice plane = axial; slice thickness: 1 mm; resolution = 1.0 × 1.0 × 1.0 mm; number of slices 176.
Data analysis
For data analyses, the Statistical Parametric Mapping analysis package (SPM12 version 7771; www.fil.ion.ucl.ac.uk/spm/software/spm12/; last access: 27/11/2020) and the computational anatomy toolbox (CAT12 version vcat12.7; dbm.neuro.uni-jena.de/cat/; last access: 27/11/2020) implemented in SPM12 for surface-based morphometry (SBM; i.e. CG and CT) were used. To ensure high data quality, all original images were subject to visual inspection, and further data quality assurance was conducted by examining sample homogeneity of individual surfaces. Participants with 2 or more standard deviations in the Mahalanobis distance were partly excluded.
Gyrification analyses were based on the absolute mean curvature (AMC) approach as implemented in CAT12 [35], which has been used in previous MRI studies analysing SZ [41, 54]. A 23 mm Full Width at Half Maximum (FWHM) smoothing to the resampled surface data for CG was applied. CT was extracted using a projection-based distance measure [14]. The resampled surface data were smoothed using an 18 mm FWHM.
To investigate the relationship between CT and CG, respectively, multiple regression analyses were performed using whole-brain data and the OSSTI scale. The two OSSTI domains (OSSTI-A and OSSTI-I) were separately investigated. Higher scores in both sub-scales refer to a better illness insight. Each model included age, gender and olanzapine equivalents (OLZe) as covariates. Overall illness severity was accounted for by including PANSS total score as a further covariate. Inference was based on a peak-level threshold of p < 0.005 (uncorrected at the voxel/vertex level), in conjunction with an empirically determined extent-threshold k based on Random Field Theory (i.e., expected voxels or vertices or cluster per contrast) based on SPM resolution elements. Stereotaxic coordinates of significant between-group differences are reported from maxima within a given cluster according to the Montreal Neurological Institute (MNI) template. Following vertex values, distinct anatomical regions emerging from the between-group comparisons were labelled according to the DK40 atlas [15].
To investigate whether cortical morphology in SZ patients differs from HC in regions exhibiting significant associations with illness insight, the two-sample t test design implemented in CAT12 was performed comparing SZ and HC using whole-brain data separately for CT and CG adjusting for age and gender (as above uncorrected threshold p < 0.005, extent-threshold = k, see above). These whole-brain analyses were complemented by a region-of-interest (ROI) based approach, after extracting CG and CT according to the DK40 atlas from regions showing a significant relationship with OSSTI-I or OSSTI-A in the aforementioned regression analysis. ROI-analyses were conducted and displayed offline using the R software environment for statistical computing (version 4.0.3; https://www.r-project.org/; last access: 15/11/2020) [55]. ANCOVAs were performed for each region separately using age and gender as covariates; if the test assumptions were not met, robust ANCOVAs were performed using age as covariate, p values were adjusted for multiple comparisons using Bonferroni correction. An uncorrected threshold of p < 0.05 was applied. For significant adjusted p values, effect sizes were calculated.
Results
Demographics and clinical scores
The groups significantly differed in age (p = 0.003, Mann–Whitney U test) and education years (p < 0.001, Mann–Whitney U test). A significant gender difference was not found (p = 0.55, Chi-Square-test). For further clinical details, see Table 1. Both OSSTI sub-scales correlated negatively with PANSS item G12, lack of judgment and insight (OSSTI-I: R = − 0.35, p = 0.0015; OSSTI-A: R = 0.28, p = 0.012, Pearson's correlation).
Associations between CG and illness insight
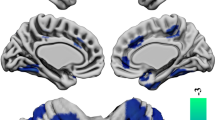
In SPM-based multiple regression analyses for OSSTI-A, significant negative association were found in the left inferior parietal lobule (IPL) and the right superior parietal gyrus, and significant positive associations were found in the right superior frontal and the precentral gyrus (M1). For OSSTI-I, significant negative associations were found in the left superior frontal gyrus and significant positive associations in the right supramarginal gyrus (see also Fig. 1 and Table 2).
Negative and positive associations for CG and OSSTI-I and OSSTI-A. Results derived from whole-brain regression analyses implemented in SPM12, adjusted for age, gender, OLZe, PANSS (p < 0.005, uncorrected, expected voxels per cluster). CG cortical gyrification, OLZe Olanzapine equivalents, OSSTI Osnabrueck Scale of Therapeutic Attitudes and Identification of Psychological Problems in Schizophrenia (OSSTI-A adherence), OSSTI-I identification of disease-related symptoms), PANSS Positive and Negative Syndrome Scale
Associations between CT and illness insight
Whole-brain regression analyses performed for OSSTI-A, revealed significant positive associations in the left IPL. For OSSTI-I, significant negative associations were found in the left pars triangularis of the inferior frontal gyrus and the right superior temporal gyrus and M1 (see also Fig. 2 and Table 3).
Negative and positive associations in the multiple regression analysis for CT and OSSTI-I and OSSTI-A. Results derived from whole-brain regression analyses implemented in SPM12, adjusted for age, gender, OLZe, PANSS (p < 0.005, uncorrected, expected voxels per cluster). CT cortical thickness, OLZe Olanzapine equivalents, OSSTI Osnabrueck Scale of Therapeutic Attitudes and Identification of Psychological Problems in Schizophrenia (OSSTI-A adherence; OSSTI-I identification of disease-related symptoms), PANSS Positive and Negative Syndrome Scale
Comparisons with healthy controls
In comparison with HC regionally specific differences were found for both CG and CT (for more details, see supplements). In brief, SZ patients had higher CG in the frontal (left superior frontal gyrus) and the temporal lobe (left transverse temporal gyrus) and the cingulate (left rostral anterior cingulate) whereas lower CG was found in the frontal lobe (right superior frontal gyrus) as well as in both insulas compared to HC. For CT, SZ patients had significant lower CT in frontal (left precentral, paracentral, medial orbitofrontal gyrus and the right M1, rostral middle frontal and superior frontal gyrus), parietal (left superior parietal and right supramarginal gyrus), temporal (left inferior temporal gyrus) and occipital (left and right lateral occipital gyrus) regions as well as the cingulate (left posterior cingulate, right caudal anterior cingulate) in comparison with HC.
Finally, in ANCOVAs, there were significant differences for CT in the left IPL (p = 0.0262, Bonferroni corrected) and pars triangularis (p = 0.0463, Bonferroni corrected) as well as right superior temporal gyrus (p = 0.0441, Bonferroni corrected; see also supplementary material).
Discussion
The main objective of this study was to examine associations between illness insight and CG and CT in SZ. We specifically focused on both symptom identification and treatment adherence. Two major findings emerged: first, we detected consistent associations between illness insight and both CG and CT in the left IPL and the right M1 only. Second, adherence and symptom identification dimensions showed distinct associations with brain morphology. In particular, the adherence domain showed significant associations with parietal (for CG: left inferior and right superior parietal gyrus (SPG); for CT left IPL) and frontal cortex (CG: right superior frontal gyrus and M1). Symptom identification was related to frontoparietal CG (right SPG, left superior frontal gyrus) as well as to CT in the parietal (right M1 and left pars triangularis) and the right superior temporal gyrus (STG).
Previous neuroimaging studies on the relationship between illness insight and brain morphology focused on GMV and CT. Although these studies used different analysis methods and yielded different results, they converged in brain regions such as frontoparietal, temporal, and cingulate cortex as well as precuneus [11] as crucial sites responsible for illness insight in SZ [13, 39, 49, 50]. Particularly noteworthy is the study by Beland et al., which did not find any correlations between CT and illness insight in SZ [6]. The authors suggested a limited role of CT and a greater role of psychological processes in the pathophysiology of poor illness insight in SZ [6]. Although the findings of abovementioned VBM studies might have been confounded by CT and cortical folding variations [32, 33, 58], thus limiting the comparability with the present study, we extend the findings of the previous studies by examining two cortical markers that show a distinct development over time, i.e. CG vs. CT.
Particularly noteworthy is the association between illness insight and CG and CT of the parietal cortex (e.g. involving IPL), suggesting both early neurodevelopmental variation as well as changes across adolescence and young adulthood. Two previous studies showed an association between illness insight and GMV and CT of the IPL [10, 13]. In particular, the IPL might play an essential role in the modulation of working memory, planning or problem solving [9, 51]. Aberrations in the non-dominant parietal gyrus additionally to frontal regions, are associated with anosognosia in numerous neurological diseases such as hemiplegia [38, 45]. The abovementioned functions may be linked to the functional coupling of the IPL within the so-called default mode network (DMN). DMN has been strongly implicated in self-reference and self-attribution [2], and its aberrant functioning has been frequently found in SZ patients [26]. CG and CT variations of the IPL highlight the relevance of this region for inter-individually varying illness insight, as much as they highlight possible similarities of illness insight in SZ and anosognosia in neurological diseases. Besides the IPL, the SPG was also found to be related to illness insight in this study. Both the IPL and SPG play an important role in executive cognition, particularly in working memory [12]. In addition, the supramarginal gyrus has been suggested to play a central role in processing information from different sensory modalities [29]. With its important role in different parts of working memory, variations in the parietal gyri might modulate sensory awareness and information processing along with subsequent decision-making, thus leading to various levels of recognition and self-attribution of a deviant health condition, i.e. SZ [23, 48] .
Furthermore, this study showed significant associations between OSSTI scores and CG in the right superior frontal, both CG and CT in M1 as well as CG in the left superior frontal gyrus and CT in pars triangularis. Since illness insight consists of at least three different dimensions, i.e., psychical, emotional, and somaesthetic [56], these findings support the neurobiological basis of this model for several reasons: first, the superior frontal region plays a crucial role in introspection, self-judgment, and self-awareness [21]. From a pathomechanistic perspective, aberrant frontoparietal structure in SZ might modulate the processes of self-reflexion/-awareness (recognition) and emotional capacities (feeling into oneself) leading to impaired illness insight [53]. For instance, Sapara et al. [49] showed that reduced GMV of superior, inferior and orbitofrontal regions are correlated with lower illness insight in SZ patients. A later study by Parellada et al. [44] corroborated these findings by showing that illness insight of early-onset first-episode psychosis patients depends on frontoparietal GMV. Interestingly, Buchy et al. [10] found misattribution of symptoms to be associated with CT in orbitofrontal cortex (OFC) and dorsolateral prefrontal cortex (DLPFC). Second, M1 variations might cause an impairment of somaesthetic body awareness apart from a less efficient control of sensorimotor functioning. Third, the pars triangularis of the inferior frontal gyrus (or ventrolateral prefrontal cortex) is responsible for two different forms of cognitive control, i.e. active retrieval and proactive interference resolution [30, 53]. Difficulties of active retrieval can have a negative impact on the process of self-reflection/awareness and lead to poor illness insight. Aberrant proactive interference resolution may contribute to misinterpretation of sensory information and difficulties to differentiate between an internal or external source of movement or sound as is known in patients with severe psychotic symptoms. These findings highlight the possibility of modulating illness insight via suppressing psychotic symptoms by antipsychotic medication or with specific psychotherapeutic interventions, which above all strengthen the cognitive control (e.g. metacognitive training) [40]. This said, decreasing the positive symptoms load and increasing the cognitive capacity (using cognitive training strategies) to attribute psychotic symptoms to an internal source may be helpful to increase illness insight and strengthen patients’ adherence. Finally, we found a negative association between CT in the right STG and OSSTI-I score. Few studies found associations between the ability to recognize experiences as abnormal [13] or direct associations between illness insight and GMV in STG [16, 39]. For instance, Buchy et al. [11] showed cortical thinning of temporal gyri in first-episode psychotic patients with insight deficits. Interestingly, fronto-temporal transcranial direct current stimulation (tDCS) optimizes attitudes toward mental illness [27]. Eventually, poor illness insight is not unique for SZ patients, but is also a well-recognized clinical phenomenon in patients with obsessive–compulsive (OCD) and eating disorders (ED). Few MRI studies explored the neurobiological origin of poor illness insight in OCD. According to Fan et al. [17], OCD patients with poor insight showed reduced amplitude of low-frequency fluctuation (ALFF) in left middle temporal gyrus and right STG, as well as increased ALFF in right middle occipital gyrus compared to OCD patients with good insight. Another fMRI study by Fan et al. [18] found decreased functional connectivity (FC) between anterior insula and medial OFC and increased FC between AI and dorsal anterior cingulate cortex (dACC) in OCD patients with poor insight compared to HC. More recently, Liu et al. [34] found that OCD patients with poor insight showed reduced CT in the left superior frontal gyrus, left ACC and right IPL, compared to HC. In ED, numerous studies focused on the neurocognitive basis of poor illness insight suggesting impaired self-awareness [3], cognitive-behavioural flexibility [61] and theory of mind [8]. Surprisingly, in sharp contrast to the data available in SZ or OCD, no MRI study so far specifically examined neural correlates of poor illness insight in ED. Given that poor illness insight is a transdiagnostic phenomenon, future studies should seek to expand extant models of illness insight well beyond SZ or OCD.
Strengths of this study include (i) the patient sample size, (ii) well-matched study groups, (iii) the use of advanced surfaced-based morphometry techniques for the investigation of cortical markers with different developmental trajectories. A potential limitation of this study is the cross-sectional study design, because illness insight is not a stable clinical feature, but depends on psychopathological symptoms and neurocognitive functioning and may fluctuate over time. Furthermore, given that CG is a rather stable neural trait, this marker may be indicative of vulnerability and risk to a higher degree than other structural features, e.g. grey matter volume or CT, that change over time as a function of age, environmental processes or disease-related factors. Nevertheless, in the light of this study’s findings, cortical surface feature of long-term temporal stability are linked to illness insight in stages of manifest disease, supporting the notion that CG abnormalities, if already present in vulnerable samples prior to manifest (first-episode) psychosis, could decisively modulate the degree of illness insight and related (long-term) clinical outcomes. This said, longitudinal studies investigating high-risk populations with both good and poor illness insight, heavy cannabis users prone to psychotic episodes and subsequent development of SZ, as well as first-degree relatives of SZ patients are needed to provide definitive conclusions.
Conclusion
The results support a multi-parametric neuronal model of illness insight in SZ patients, suggesting an impact of working memory, introspection, self-awareness as well as sensory information processing. Especially, the IPL as part of the DMN might play an important role in the onset of inter-individually varying illness insight. As we found significant associations between both CG and CT, illness insight might be influenced by a neurobiological vulnerability, environmental influences in the brain development after birth as well as later structural brain changes, caused by the disease itself but also by other modulating factors such as antipsychotic medication [24]. This might provide opportunities to positively influence illness insight during the diseases progress—with antipsychotic medication or specific psychotherapeutic trainings.
Availability of data and material
Data will be made available on scientifically reasonable request. Material will be made available on scientifically reasonable request.
Code availability
Data will be made available on scientifically reasonable request.
Abbreviations
- ALFF:
-
Amplitude of low-frequency fluctuation
- AMC:
-
Absolute mean curvature
- BIS:
-
Birchwood’s Insight Scale
- CAT12:
-
Computational Anatomy Toolbox
- CT:
-
Cortical thickness
- CG:
-
Cortical gyrification
- dACC:
-
Dorsal anterior cingulate cortex
- DK40:
-
Desikan–Killiany atlas
- DLPFC:
-
Dorsolateral prefrontal cortex
- DMN:
-
Default mode network
- ED:
-
Eating disorder
- FC:
-
Functional connectivity
- FDR:
-
False discovery rate
- FOV:
-
Field of view
- FWHM:
-
Full width at half maximum
- GMV:
-
Grey matter volume
- HC:
-
Healthy controls
- IPL:
-
Inferior parietal lobule
- MNI:
-
Montreal Neurological Institute
- MRI:
-
Magnetic resonance imaging
- M1:
-
Precentral gyrus
- OCD:
-
Obsessive-compulsive disorder
- OFC:
-
Orbitofrontal cortex
- OLZe:
-
Olanzapine equivalents
- OSSTI:
-
Osnabrueck Scale of Therapeutic Attitudes and Identification of Psychological Problems in Schizophrenia (originally in German: Osnabrücker Skala zu Therapieeinstellung und Identifikation psychischer Beschwerden bei Schizophrenie)
- OSSTI-A:
-
Adherence domain of OSSTI
- OSSTI-I:
-
Identification of disease-related symptoms domain of OSSTI
- PANSS:
-
Positive and Negative Syndrome Scale
- ROI:
-
Region of interest
- SAIQ:
-
“Self-Appraisal of Illness Questionnaire”
- SBM:
-
Surface-based morphometry
- SPG:
-
Superior parietal gyrus
- SPM12:
-
Statistical parametric mapping
- STG:
-
Superior temporal gyrus
- SUMD:
-
Scale to assess unawareness of mental disorder
- SZ:
-
Patients with schizophrenia
- tDCS:
-
Fronto-temporal transcranial direct current stimulation
- TE:
-
Echo time
- TI:
-
Inversion time
- TR:
-
Repetition time
- VBM:
-
Voxel-based morphometry
References
Amador XF, Strauss DH, Yale SA, Flaum MM, Endicott J, Gorman JM (1993) Assessment of insight in psychosis. Am J Psychiatry 150:873–873
Andrews-Hanna JR, Reidler JS, Huang C, Buckner RL (2010) Evidence for the default network’s role in spontaneous cognition. J Neurophysiol 104:322–335
Arbel R, Koren D, Klein E, Latzer Y (2013) The neurocognitive basis of insight into illness in anorexia nervosa: a pilot metacognitive study. Psychiatry Res 209:604–610
Armstrong E, Schleicher A, Omran H, Curtis M, Zilles K (1995) The ontogeny of human gyrification. Cereb Cortex 5:56–63
Bassitt DP, Neto MR, de Castro CC, Busatto GF (2007) Insight and regional brain volumes in schizophrenia. Eur Arch Psychiatry Clin Neurosci 257:58–62
Beland S, Makowski C, Konsztowicz S, Buchy L, Chakravarty MM, Lepage M (2019) Clarifying associations between cortical thickness, subcortical structures, and a comprehensive assessment of clinical insight in enduring schizophrenia. Schizophr Res 204:245–252
Birchwood M, Smith J, Drury V, Healy J, Macmillan F, Slade M (1994) A self-report insight scale for psychosis: reliability, validity and sensitivity to change. Acta Psychiatr Scand 89:62–67
Bora E, Köse S (2016) Meta-analysis of theory of mind in anorexia nervosa and bulimia nervosa: a specific impairment of cognitive perspective taking in anorexia nervosa? Int J Eat Disord 49:739–740
Bublak P, Müller U, Grön G, Reuter M, von Cramon DY (2002) Manipulation of working memory information is impaired in Parkinson’s disease and related to working memory capacity. Neuropsychology 16:577
Buchy L, Ad-Dab’bagh Y, Lepage C, Malla A, Joober R, Evans A, Lepage M (2012) Symptom attribution in first episode psychosis: a cortical thickness study. Psychiatry Res Neuroimag 203:6–13
Buchy L, Ad-Dab’bagh Y, Malla A, Lepage C, Bodnar M, Joober R, Sergerie K, Evans A, Lepage M (2011) Cortical thickness is associated with poor insight in first-episode psychosis. J Psychiatr Res 45:781–787
Cabeza R, Ciaramelli E, Olson IR, Moscovitch M (2008) The parietal cortex and episodic memory: an attentional account. Nat Rev Neurosci 9:613–625
Cooke MA, Fannon D, Kuipers E, Peters E, Williams SC, Kumari V (2008) Neurological basis of poor insight in psychosis: a voxel-based MRI study. Schizophr Res 103:40–51
Dahnke R, Yotter RA, Gaser C (2013) Cortical thickness and central surface estimation. Neuroimage 65:336–348
Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, Albert MS, Killiany RJ (2006) An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 31:968–980
Emami S, Guimond S, Mallar Chakravarty M, Lepage M (2016) Cortical thickness and low insight into symptoms in enduring schizophrenia. Schizophr Res 170:66–72
Fan J, Zhong M, Gan J, Liu W, Niu C, Liao H, Zhang H, Tan C, Yi J, Zhu X (2017) Spontaneous neural activity in the right superior temporal gyrus and left middle temporal gyrus is associated with insight level in obsessive-compulsive disorder. J Affect Disord 207:203–211
Fan J, Zhong M, Zhu X, Gan J, Liu W, Niu C, Liao H, Zhang H, Yi J, Tan C (2017) Resting-state functional connectivity between right anterior insula and right orbital frontal cortex correlate with insight level in obsessive-compulsive disorder. NeuroImage Clin 15:1–7
Fernández V, Llinares-Benadero C, Borrell V (2016) Cerebral cortex expansion and folding: what have we learned? EMBO J 35:1021–1044
Giedd JN, Rapoport JL (2010) Structural MRI of pediatric brain development: what have we learned and where are we going? Neuron 67:728–734
Goldberg II, Harel M, Malach R (2006) When the brain loses its self: prefrontal inactivation during sensorimotor processing. Neuron 50:329–339
Habets P, Marcelis M, Gronenschild E, Drukker M, van Os J (2011) Reduced cortical thickness as an outcome of differential sensitivity to environmental risks in schizophrenia. Biol Psychiat 69:487–494
Hahn B, Robinson BM, Leonard CJ, Luck SJ, Gold JM (2018) Posterior parietal cortex dysfunction is central to working memory storage and broad cognitive deficits in schizophrenia. J Neurosci 38(39):8378–8387. https://doi.org/10.1523/JNEUROSCI.0913-18.2018
Ho B-C, Andreasen NC, Ziebell S, Pierson R, Magnotta V (2011) Long-term antipsychotic treatment and brain volumes: a longitudinal study of first-episode schizophrenia. Arch Gen Psychiatry 68:128–137
Hogstrom LJ, Westlye LT, Walhovd KB, Fjell AM (2013) The structure of the cerebral cortex across adult life: age-related patterns of surface area, thickness, and gyrification. Cereb Cortex 23:2521–2530
Hu M-L, Zong X-F, Mann JJ, Zheng J-J, Liao Y-H, Li Z-C, He Y, Chen X-G, Tang J-S (2017) A review of the functional and anatomical default mode network in schizophrenia. Neurosci Bull 33:73–84
Kao Y-C, Tzeng N-S, Chao C-Y, Chang C-C, Chang H-A (2020) Modulation of self-appraisal of illness, medication adherence, life quality and autonomic functioning by transcranial direct current stimulation in schizophrenia patients. Clin Neurophysiol 131:1997–2007
Kay SR, Opler LA, Lindenmayer J-P (1989) The positive and negative syndrome scale (panss): rationale and standardisation. Br J Psychiatry 155:59–65
Kheradmand A, Lasker A, Zee DS (2015) Transcranial magnetic stimulation (tms) of the supramarginal gyrus: a window to perception of upright. Cereb Cortex 25:765–771
Kostopoulos P, Petrides M (2008) Left mid-ventrolateral prefrontal cortex: underlying principles of function. Eur J Neurosci 27:1037–1049
Krupa T (2005) Krankheitseinsicht, copingstrategien und neurokognitive beeinträchtigungen bei schizophrenen patienten. Unveröffentlichte Diplomarbeit, Universität Osnabrück, Osnabrück
Kurth F, Luders E, Gaser C (2015) Voxel-based morphometry. Elsevier, Amsterdam
Lemaitre H, Goldman AL, Sambataro F, Verchinski BA, Meyer-Lindenberg A, Weinberger DR, Mattay VS (2012) Normal age-related brain morphometric changes: nonuniformity across cortical thickness, surface area and gray matter volume? Neurobiol Aging 33:617.e611-617.e619
Liu W, Gan J, Fan J, Zheng H, Li S, Chan RC, Tan C, Zhu X (2019) Associations of cortical thickness, surface area and subcortical volumes with insight in drug-naive adults with obsessive-compulsive disorder. NeuroImage Clin 24:102037
Luders E, Thompson PM, Narr KL, Toga AW, Jancke L, Gaser C (2006) A curvature-based approach to estimate local gyrification on the cortical surface. Neuroimage 29:1224–1230
Lysaker PH, Pattison ML, Leonhardt BL, Phelps S, Vohs JL (2018) Insight in schizophrenia spectrum disorders: relationship with behavior, mood and perceived quality of life, underlying causes and emerging treatments. World Psychiatry 17:12–23
Marks KA, Fastenau PS, Lysaker PH, Bond GR (2000) Self-appraisal of illness questionnaire (saiq): relationship to researcher-rated insight and neuropsychological function in schizophrenia. Schizophr Res 45:203–211
McGlynn SM, Schacter DL (1989) Unawareness of deficits in neuropsychological syndromes. J Clin Exp Neuropsychol 11:143–205
Morgan KD, Dazzan P, Morgan C, Lappin J, Hutchinson G, Suckling J, Fearon P, Jones PB, Leff J, Murray RM, David AS (2010) Insight, grey matter and cognitive function in first-onset psychosis. Br J Psychiatry 197:141–148
Moritz S, Andreou C, Schneider BC, Wittekind CE, Menon M, Balzan RP, Woodward TS (2014) Sowing the seeds of doubt: a narrative review on metacognitive training in schizophrenia. Clin Psychol Rev 34:358–366
Nenadic I, Maitra R, Dietzek M, Langbein K, Smesny S, Sauer H, Gaser C (2015) Prefrontal gyrification in psychotic bipolar I disorder vs. Schizophrenia. J Affect Disord 185:104–107
Oldfield RC (1971) The assessment and analysis of handedness: the edinburgh inventory. Neuropsychologia 9:97–113
Olivares JM, Sermon J, Hemels M, Schreiner A (2013) Definitions and drivers of relapse in patients with schizophrenia: a systematic literature review. Ann Gen Psychiatry 12:1–11
Parellada M, Boada L, Fraguas D, Reig S, Castro-Fornieles J, Moreno D, Gonzalez-Pinto A, Otero S, Rapado-Castro M, Graell M (2011) Trait and state attributes of insight in first episodes of early-onset schizophrenia and other psychoses: a 2-year longitudinal study. Schizophr Bull 37:38–51
Pia L, Neppi-Modona M, Ricci R, Berti A (2004) The anatomy of anosognosia for hemiplegia: a meta-analysis. Cortex 40:367–377
Raichle ME (2015) The brain’s default mode network. Annu Rev Neurosci 38:433–447
Rakic P (2009) Evolution of the neocortex: a perspective from developmental biology. Nat Rev Neurosci 10:724–735
Rohleder C, Koethe D, Fritze S, Topor CE, Leweke FM, Hirjak D (2019) Neural correlates of binocular depth inversion illusion in antipsychotic-naïve first-episode schizophrenia patients. Eur Arch Psychiatry Clin Neurosci 269(8):897–910. https://doi.org/10.1007/s00406-018-0886-2
Sapara A, Cooke M, Fannon D, Francis A, Buchanan RW, Anilkumar AP, Barkataki I, Aasen I, Kuipers E, Kumari V (2007) Prefrontal cortex and insight in schizophrenia: a volumetric MRI study. Schizophr Res 89:22–34
Sapara A, Ffytche DH, Cooke MA, Williams SC, Kumari V (2016) Voxel-based magnetic resonance imaging investigation of poor and preserved clinical insight in people with schizophrenia. World J Psychiatry 6:311
Shad MU, Keshavan MS, Tamminga CA, Cullum CM, David A (2007) Neurobiological underpinnings of insight deficits in schizophrenia. Int Rev Psychiatry (Abingdon, Engl) 19:437–446
Smith G, Thornton A, Lang D, MacEwan G, Kopala L, Su W, Honer W (2015) Cortical morphology and early adverse birth events in men with first-episode psychosis. Psychol Med 45:1825–1837
Spalletta G, Piras F, Piras F, Caltagirone C, Orfei MD (2014) The structural neuroanatomy of metacognitive insight in schizophrenia and its psychopathological and neuropsychological correlates. Hum Brain Mapp 35:4729–4740
Spalthoff R, Gaser C, Nenadić I (2018) Altered gyrification in schizophrenia and its relation to other morphometric markers. Schizophr Res 202:195–202
Team RC (2020) R: a language and environment for statistical computing
Thirioux B, Harika-Germaneau G, Langbour N, Jaafari N (2020) The relation between empathy and insight in psychiatric disorders: phenomenological, etiological, and neuro-functional mechanisms. Front Psych 10:966
Turnbull OH, Fotopoulou A, Solms M (2014) Anosognosia as motivated unawareness: the ‘defence’ hypothesis revisited. Cortex 61:18–29
Voets NL, Hough MG, Douaud G, Matthews PM, James A, Winmill L, Webster P, Smith S (2008) Evidence for abnormalities of cortical development in adolescent-onset schizophrenia. Neuroimage 43:665–675
Waldorf M (2010) Krankheitseinsicht, dynamisch getestete exekutivfunktionen und defensive bewältigung bei schizophrenie. Unveröffentlichte Dissertation, Universität Osnabrück
Whitfield-Gabrieli S, Ford JM (2012) Default mode network activity and connectivity in psychopathology. Annu Rev Clin Psychol 8:49–76
Zastrow A, Kaiser S, Stippich C, Walther S, Herzog W, Tchanturia K, Belger A, Weisbrod M, Treasure J, Friederich H-C (2009) Neural correlates of impaired cognitive-behavioral flexibility in anorexia nervosa. Am J Psychiatry 166:608–616
Zilles K, Palomero-Gallagher N, Amunts K (2013) Development of cortical folding during evolution and ontogeny. Trends Neurosci 36:275–284
Funding
Open Access funding enabled and organized by Projekt DEAL. This work was supported by the German Research Foundation (DFG) (Grant number DFG HI 1928/2-1 to D.H. and WO 1883/6-1 and WO 1883/15-1 to R.C.W.) and German Federal Ministry of Education and Research (BMBF, grant 01GQ1102 to H.T.). The DFG and BMBF had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Author information
Authors and Affiliations
Contributions
DH, US and RCW designed the study. DH, RCW and HT obtained funding. DH, SF, LSG, and HT recruited, assessed, and scanned subjects. MLO and MMS performed statistical analysis. RCW and MMS supervised analyses. NDW, KMK, and AML interpreted and critically discussed the results. MLO, RCW and DH wrote the first draft of the manuscript. All authors contributed to and approved the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have declared that there are no conflicts of interest in relation to the subject of this study.
Ethics approval
The local ethics committee (Medical Faculty at Heidelberg University, Germany) approved the study.
Consent for publication
All patients gave informed consent to participate in the study and for publication.
Ethical standards
All procedures contributing to this work complied with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised 2008. The local ethics committee (Medical Faculty at Heidelberg University, Germany) approved the study. Written informed consent was obtained from all participants following a complete description of the study.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Otte, ML., Schmitgen, M.M., Kubera, K.M. et al. Cortical morphology and illness insight in patients with schizophrenia. Eur Arch Psychiatry Clin Neurosci 272, 985–995 (2022). https://doi.org/10.1007/s00406-021-01328-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00406-021-01328-x