Abstract
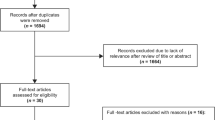
The objective is to understand genetic predisposition to delirium. Following PRISMA guidelines, we undertook a systematic review of studies involving delirium and genetics in the databases of Pubmed, Scopus, Cochrane Library and PsycINFO, and performed a meta-analysis when appropriate. We evaluated 111 articles, of which 25 were finally included in the analysis. The studies were assessed by two independent researchers for methodological quality using the Downs and Black Tool and for genetic analysis quality. We performed a meta-analysis of 10 studies of the Apolipoprotein E (APOE) gene, obtaining no association with the presence of delirium (LOR 0.18, 95% CI − 0.10–0.47, p = 0.21). Notably, only 5 out of 25 articles met established criteria for genetic studies (good quality) and 6 were of moderate quality. Seven studies found an association with APOE4, the dopamine transporter gene SCL6A3, dopamine receptor 2 gene, glucocorticoid receptor, melatonin receptor and mitochondrial DNA haplotypes. One genome-wide association study found two suggestive long intergenic non-coding RNA genes. Five studies found no association with catechol-o-methyltransferase, melatonin receptor or several interleukins genes. The studies were heterogenous in establishing the presence of delirium. Future studies with large samples should further specify the delirium phenotype and deepen our understanding of interactions between genes and other biological factors.



Similar content being viewed by others
Availability of data and materials
All data generated or analysed during this study are included within this article (and its supplementary information files).
References
Ryan DJ, O’Regan NA, Caoimh RO, Clare J, O’Connor M, Leonard M et al (2013) Delirium in an adult acute hospital population: predictors, prevalence and detection. BMJ Open 3:e001772
Kalish VB, Gillham JE, Unwin BK (2014) Delirium in older persons: evaluation and management. Am Fam Physician 90:150–158
Witlox J, Eurelings LS, de Jonghe JF, Kalisvaart KJ, Eikelenboom P, van Gool WA (2010) Delirium in elderly patients and the risk of postdischarge mortality, institutionalization, and dementia: a meta-analysis. JAMA 304:443–451
Siddiqi N, House AO, Holmes JD (2006) Occurrence and outcome of delirium in medical in-patients: a systematic literature review. Age Ageing 35:350–364
Sepulveda E, Franco JG, Trzepacz PT, Gaviria AM, Meagher DJ, Palma J et al (2016) Delirium diagnosis defined by cluster analysis of symptoms versus diagnosis by DSM and ICD criteria: diagnostic accuracy study. BMC Psychiatry 16:167
Trzepacz PT (2000) Is there a final common neural pathway in delirium? Focus on acetylcholine and dopamine. Semin Clin Neuropsychiatry 5:132–148
Maldonado JR (2018) Delirium pathophysiology: an updated hypothesis of the etiology of acute brain failure. Int J Geriatr Psychiatry 33:1428–1457
Adamis D, Van Munster BC, Macdonald AJD (2009) The genetics of deliria. Int Rev Psychiatry 21:20–29
Inouye SK, Westendorp RGJ, Saczynski JS (2014) Delirium in elderly people. Lancet 383:911–922
van Munster BC, Korevaar JC, Zwinderman AH, Leeflang MM, de Rooij SEJA (2009) The association between delirium and the apolipoprotein E epsilon 4 allele: new study results and a meta-analysis. Am J Geriatr Psychiatry 17:856–862
Adamis D, Meagher D, Williams J, Mulligan O, McCarthy G (2016) A systematic review and meta-analysis of the association between the apolipoprotein E genotype and delirium. Psychiatr Genet 26:53–59
Moher D, Liberati A, Tetzlaff J, Altman DG (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 6:e1000097
Downs SH, Black N (1998) The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health 52:377–384
Hootman JM, Driban JB, Sitler MR, Harris KP, Cattano NM (2011) Reliability and validity of three quality rating instruments for systematic reviews of observational studies. Res Synth Methods 2:110–118
Hooper P, Jutai JW, Strong G, Russell-Minda E (2008) Age-related macular degeneration and low-vision rehabilitation: a systematic review. Can J Ophthalmol 43:180–187
Silverman SR, Schertz LA, Yuen HK, Lowman JD, Bickel CS (2012) Systematic review of the methodological quality and outcome measures utilized in exercise interventions for adults with spinal cord injury. Spinal Cord 50:718–727
Trac MH, McArthur E, Jandoc R, Dixon SN, Nash DM, Hackam DG et al (2016) Macrolide antibiotics and the risk of ventricular arrhythmia in older adults. CMAJ 188:e120–e129
Jorgensen TJ, Ruczinski I, Kessing B, Smith MW, Shugart YY, Alberg AJ (2009) Hypothesis-driven candidate gene association studies: practical design and analytical considerations. Am J Epidemiol 170:986–993
Hong EP, Park JW (2012) Sample size and statistical power calculation in genetic association studies. Genomics Inform 10:117
Viechtbauer W (2010) Conducting meta-analyses in R with the metafor. J Stat Softw 36:1–48
Wan X, Wang W, Liu J, Tong T (2014) Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol 14:135
Mahanna-Gabrielli E, Miano TA, Augoustides JG, Kim C, Bavaria JE, Kofke WA (2018) Does the melatonin receptor 1B gene polymorphism have a role in postoperative delirium? PLoS ONE 13:e0207941
Samuels DC, Hulgan T, Fessel JP, Billings FT, Thompson JL, Chandrasekhar R et al (2019) Mitochondrial DNA haplogroups and delirium during sepsis. Crit Care Med 47:1065–1071
van Munster BC, de Rooij SEJA, Yazdanpanah M, Tienari PJ, Pitkala KH, Osse RJ et al (2010) The association of the dopamine transporter gene and the dopamine receptor 2 gene with delirium, a meta-analysis. Am J Med Genet B Neuropsychiatr Genet 153B:648–655
Vasunilashorn SM, Ngo L, Kosar CM, Fong TG, Jones RN, Inouye SK et al (2015) Does apolipoprotein E genotype increase risk of postoperative delirium? Am J Geriatr Psychiatry 23:1029–1037
Vasunilashorn SM, Ngo LH, Jones RN, Inouye SK, Hall KT, Gallagher J et al (2019) The association between C-reactive protein and postoperative delirium differs by catechol-O-methyltransferase genotype. Am J Geriatr Psychiatry 27:1–8
Ely EW, Girard TD, Shintani AK, Jackson JC, Gordon SM, Thomason JWW et al (2007) Apolipoprotein E4 polymorphism as a genetic predisposition to delirium in critically ill patients. Crit Care Med 35:112–117
Tagarakis GI, Tsolaki-Tagaraki F, Tsolaki M, Diegeler A, Tsilimingas NB, Papassotiropoulos A (2007) The role of apolipoprotein E in cognitive decline and delirium after bypass heart operations. Am J Alzheimers Dis Other Demen 22:223–228
Tagarakis GI, Tsolaki-Tagaraki F, Tsolaki M, Diegeler A, Kazis D, Rouska E et al (2007) The role of SOAT-1 polymorphisms in cognitive decline and delirium after bypass heart surgery. Clin Res Cardiol 96:600–603
Leung JM, Sands LP, Wang Y, Poon A, Kwok P, Kane JP et al (2007) Apolipoprotein E e4 allele increases the risk of early postoperative delirium in older patients undergoing noncardiac surgery. Anesthesiology 107:406–411
Skrobik Y, Leger C, Cossette M, Michaud V, Turgeon J (2013) Factors predisposing to coma and delirium: fentanyl and midazolam exposure; CYP3A5, ABCB1, and ABCG2 genetic polymorphisms; and inflammatory factors. Crit Care Med 41:999–1008
Nekrosius D, Kaminskaite M, Jokubka R, Pranckeviciene A, Lideikis K, Tamasauskas A et al (2019) Association of COMT Val158Met polymorphism with delirium risk and outcomes after traumatic brain injury. J Neuropsychiatry Clin Neurosci 31:298–305
Westphal S, Stoppe C, Gruenewald M, Bein B, Renner J, Cremer J et al (2019) Genome-wide association study of myocardial infarction, atrial fibrillation, acute stroke, acute kidney injury and delirium after cardiac surgery—a sub-analysis of the RIPHeart-study. BMC Cardiovasc Disord. https://doi.org/10.1186/s12872-019-1002-x
Adamis D, Treloar A, Martin FC, Gregson N, Hamilton G, Macdonald AJD (2007) APOE and cytokines as biological markers for recovery of prevalent delirium in elderly medical inpatients. Int J Geriatr Psychiatry 22:688–694
Bryson GL, Wyand A, Wozny D, Rees L, Taljaard M, Nathan H (2011) A prospective cohort study evaluating associations among delirium, postoperative cognitive dysfunction, and apolipoprotein E genotype following open aortic repair. Can J Anaesth 58:246–255
Abelha FJ, Fernandes V, Botelho M, Santos P, Santos A, Machado JC et al (2012) Apolipoprotein E e4 allele does not increase the risk of early postoperative delirium after major surgery. J Anesth. https://doi.org/10.1007/s00540-012-1326-5
Oldenbeuving AW, de Kort PLM, Kappelle LJ, van Duijn CM, Roks G (2013) Delirium in the acute phase after stroke and the role of the apolipoprotein E gene. Am J Geriatr Psychiatry 21:935–937
Alexander SA, Ren D, Gunn SR, Kochanek PM, Tate J, Ikonomovic M et al (2014) Interleukin 6 and apolipoprotein E as predictors of acute brain dysfunction and survival in critical care patients. Am J Crit Care 23:49–57
Cunningham EL, Mawhinney T, Beverland D, O’Brien S, McAuley DF, Cairns R et al (2017) Observational cohort study examining apolipoprotein E status and preoperative neuropsychological performance as predictors of post-operative delirium in an older elective arthroplasty population. Age Ageing 46:779–786
van Munster BC, Yazdanpanah M, Tanck MWT, de Rooij SEJA, van de Giessen E, Sijbrands EJG et al (2010) Genetic polymorphisms in the DRD2, DRD3, and SLC6A3 gene in elderly patients with delirium. Am J Med Genet B Neuropsychiatr Genet 153B:38–45
van Munster BC, Baas F, Tanck MW, de Rooij SEJA (2011) Polymorphisms in the catechol-o-methyltransferase gene and delirium in the elderly. Dement Geriatr Cogn Disord 31:358–362
Kazmierski J, Sieruta M, Banys A, Jaszewski R, Sobow T, Liberski P et al (2014) The assessment of the T102C polymorphism of the 5HT2a receptor gene, 3723G/A polymorphism of the NMDA receptor 3A subunit gene (GRIN3A) and 421C/A polymorphism of the NMDA receptor 2B subunit gene (GRIN2B) among cardiac surgery patients with and without d. Gen Hosp Psychiatry 36:753–756
van Munster BC, Zwinderman AH, de Rooij SE (2011) Genetic variations in the interleukin-6 and interleukin-8 genes and the interleukin-6 receptor gene in delirium. Rejuvenation Res 14:425–428
de Jonghe A, de Rooij S, Tanck MWT, Sijbrands EJG, van Munster BCV (2012) Polymorphisms in the melatonin receptor 1B gene and the risk of delirium. Dement Geriatr Cogn Disord 33:306–310
Manenschijn L, van Rossum EF, Jetten AM, de Rooij SE, van Munster BC (2011) Glucocorticoid receptor haplotype is associated with a decreased risk of delirium in the elderly. Am J Med Genet B Neuropsychiatr Genet 156B:316–321
Massimo L, Munoz E, Hill N, Mogle J, Mulhall P, McMillan CT et al (2017) Genetic and environmental factors associated with delirium severity in older adults with dementia. Int J Geriatr Psychiatry 32:574–581
Adamis D, Lunn M, Martin FC, Treloar A, Gregson N, Hamilton G et al (2009) Cytokines and IGF-I in delirious and non-delirious acutely ill older medical inpatients. Age Ageing 38:326–332 (discussion 251)
Area-Gomez E, Guardia-Laguarta C, Schon EA, Przedborski S (2019) Mitochondria, OxPhos, and neurodegeneration: cells are not just running out of gas. J Clin Invest. https://doi.org/10.1172/JCI120848
McCoy TH Jr, Hart K, Pellegrini A, Perlis RH, McCoy THJ, Hart K et al (2018) Genome-wide association identifies a novel locus for delirium risk. Neurobiol Aging 68:160.e9-160.e14
Vasunilashorn SM, Ngo LH, Inouye SK, Fong TG, Jones RN, Dillon ST et al (2020) Apolipoprotein E genotype and the association between C-reactive protein and postoperative delirium: importance of gene-protein interactions. Alzheimer’s Dement 16:572–580
Adamis D, Meagher D, Treloar A, Dunne C, Larvin M, Martin FC et al (2014) Phenomenological and biological correlates of improved cognitive function in hospitalized elderly medical inpatients. Arch Gerontol Geriatr 59:593–598
Adamis D, Rooney S, Meagher D, Mulligan O, McCarthy G (2015) A comparison of delirium diagnosis in elderly medical inpatients using the CAM, DRS-R98, DSM-IV and DSM-5 criteria. Int Psychogeriatr. https://doi.org/10.1017/S1041610214002853
Sepulveda E, Franco JG, Trzepacz PT, Gaviria AM, Viñuelas E, Palma J et al (2015) Performance of the delirium rating scale-revised-98 against different delirium diagnostic criteria in a population with a high prevalence of dementia. Psychosomatics 56:530–541
Bowman K, Jones L, Pilling LC, Delgado J, Kuchel GA, Ferrucci L et al (2019) Vitamin D levels and risk of delirium. Neurology 92:e1387–e1394
Meagher DJ, Leonard M, Donnelly S, Conroy M, Adamis D, Trzepacz PT (2011) A longitudinal study of motor subtypes in delirium: relationship with other phenomenology, etiology, medication exposure and prognosis. J Psychosom Res 71:395–403
Sepulveda E, Leonard M, Franco JGJG, Adamis D, McCarthy G, Dunne C et al (2017) Subsyndromal delirium compared with delirium, dementia, and subjects without delirium or dementia in elderly general hospital admissions and nursing home residents. Alzheimer’s Dement Diagnosis Assess Dis Monit 7:1–10
Oldham MA, Flaherty JH, Maldonado JR (2018) Refining delirium: a transtheoretical model of delirium disorder with preliminary neurophysiologic subtypes. Am J Geriatr Psychiatry 26:913–924
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
ES and EV developed the article’s concept; ES and DA performed the article search; ES, DA and DM performed the methodological quality analysis; EV and SA performed the genetic quality analysis; DA performed the meta-analysis; ES and DA wrote a first draft of the manuscript; all the authors critically revised the work and all the authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Consent to participate
The manuscript does not contain clinical studies or patient data.
Ethical approval
The manuscript does not contain clinical studies or patient data.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sepulveda, E., Adamis, D., Franco, J.G. et al. The complex interaction of genetics and delirium: a systematic review and meta-analysis. Eur Arch Psychiatry Clin Neurosci 271, 929–939 (2021). https://doi.org/10.1007/s00406-021-01255-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00406-021-01255-x