Abstract
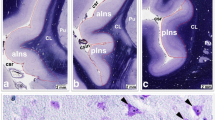
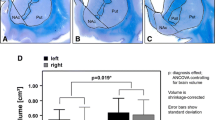
The Habenula is increasingly being investigated in addiction. Reduced volumes of other relevant brain regions in addiction, such as nucleus accumbens, globus pallidus and hypothalamus have been reported. Reduced volumes of the habenula as well as reduced neuronal cell count in the habenula have also been reported in mood disorders and an overlap between mood disorders and addiction is clinically widely recognized. Thus, our aim was to investigate possible volume and neuronal cell count differences in heroin addicts compared to healthy controls. Volumes of the medial (MHB) and lateral habenula (LHB) in heroin addicts (n = 12) and healthy controls (n = 12) were assessed by morphometry of 20 µm serial whole brain sections. Total brain volume was larger in the heroin group (mean 1466.6 ± 58.5 cm3 vs. mean 1331.5 ± 98.8 cm3), possibly because the heroin group was about 15 years younger (p = 0.001). Despite larger mean whole brain volume, the mean relative volume of the MHB was smaller than in healthy non-addicted controls (6.94 ± 2.38 × 10–6 vs.10.64 ± 3.22 × 10−6; p = 0.004). A similar finding was observed regarding relative volumes of the LHB (46.62 ± 10.90 × 10−6 vs. 63.05 ± 16.42 × 10−6 p = 0.009). In parallel, neuronal cell numbers were reduced in the MHB of heroin-addicted subjects (395,966 ± 184,178 vs. 644,149 ± 131,140; p < 0.001). These findings were not significantly confounded by age and duration of autolysis. Our results provide further evidence for brain-structural deficits in heroin addiction.


Similar content being viewed by others
References
Degenhardt L, Charlson F, Mathers B, Hall WD, Flaxman AD, Johns N, Vos T (2014) The global epidemiology and burden of opioid dependence: results from the global burden of disease 2010 study. Addict (Abingdon Engl). https://doi.org/10.1111/add.12551
Upp LA, Waljee JF (2020) The opioid epidemic. Clin Plast Surg 47:181–190. https://doi.org/10.1016/j.cps.2019.12.005
Patterson Silver Wolf DA, Gold M (2020) Treatment resistant opioid use disorder (TROUD): definition, rationale, and recommendations. J Neurol Sci 411:116718–116815. https://doi.org/10.1016/j.jns.2020.116718
Müller UJ, Truebner K, Schiltz K, Kuhn J, Mawrin C, Dobrowolny H, Bernstein H-G, Bogerts B, Steiner J (2015) Postmortem volumetric analysis of the nucleus accumbens in male heroin addicts: implications for deep brain stimulation. Eur Arch Psychiatry Clin Neurosci 265:647–653. https://doi.org/10.1007/s00406-015-0617-x
Müller UJ, Mawrin C, Frodl T, Dobrowolny H, Busse S, Bernstein H-G, Bogerts B, Truebner K, Steiner J (2019) Reduced volumes of the external and internal globus pallidus in male heroin addicts: a postmortem study. Eur Arch Psychiatry Clin Neurosci 269:317–324. https://doi.org/10.1007/s00406-018-0939-6
Seifert CL, Magon S, Sprenger T, Lang UE, Huber CG, Denier N, Vogel M, Schmidt A, Radue E-W, Borgwardt S, Walter M (2015) Reduced volume of the nucleus accumbens in heroin addiction. Eur Arch Psychiatry Clin Neurosci 265:637–645. https://doi.org/10.1007/s00406-014-0564-y
Müller UJ, Schiltz K, Mawrin C, Dobrowolny H, Frodl T, Bernstein H-G, Bogerts B, Truebner K, Steiner J (2017) Total hypothalamic volume is reduced in postmortem brains of male heroin addicts. Eur Arch Psychiatry Clin Neurosci. https://doi.org/10.1007/s00406-017-0809-7
Bielau H, Trübner K, Krell D, Agelink MW, Bernstein HG, Stauch R, Mawrin C, Danos P, Gerhard L, Bogerts B, Baumann B (2005) Volume deficits of subcortical nuclei in mood disorders. Eur Arch Psychiatry Clin Neurosci 255:401–412. https://doi.org/10.1007/s00406-005-0581-y
Zippel-Schultz B, Specka M, Cimander K, Eschenhagen T, Golz J, Maryschok M, Nowak M, Poehlke T, Stover H, Helms TM, Scherbaum N (2016) Outcomes of patients in long-term opioid maintenance treatment. Subst Use Misuse 51:1493–1503. https://doi.org/10.1080/10826084.2016.1188946
Ranft K, Dobrowolny H, Krell D, Bielau H, Bogerts B, Bernstein HG (2009) Evidence for structural abnormalities of the human habenular complex in affective disorders but not in schizophrenia. Psychol Med 40:557–567. https://doi.org/10.1017/s0033291709990821
Roman E, Weininger J, Lim B, Roman M, Barry D, Tierney P, O’Hanlon E, Levins K, O’Keane V, Roddy D (2020) Untangling the dorsal diencephalic conduction system: a review of structure and function of the stria medullaris, habenula and fasciculus retroflexus. Brain Struc Func. https://doi.org/10.1007/s00429-020-02069-8
Boulos L-J, Darcq E, Kieffer BL (2017) Translating the habenula-from rodents to humans. Biol Psychiat 81:296–305. https://doi.org/10.1016/j.biopsych.2016.06.003
Sartorius A, Henn FA (2007) Deep brain stimulation of the lateral habenula in treatment resistant major depression. Med Hypotheses 69:1305–1308. https://doi.org/10.1016/j.mehy.2007.03.021
Kiening K, Sartorius A (2013) A new translational target for deep brain stimulation to treat depression. EMBO Mol Med 5:1151–1153. https://doi.org/10.1002/emmm.201302947
Hoyer C, Kranaster L, Sartorius A, Hellweg R, Gass P (2012) Long-term course of brain-derived neurotrophic factor serum levels in a patient treated with deep brain stimulation of the lateral habenula. Neuropsychobiology 65:147–152. https://doi.org/10.1159/000335243
Meye FJ, Trusel M, Soiza-Reilly M, Mameli M (2017) Neural circuit adaptations during drug withdrawal—Spotlight on the lateral habenula. Pharmacol Biochem Behav 162:87–93. https://doi.org/10.1016/j.pbb.2017.08.007
Mathis V, Kenny PJ (2018) From controlled to compulsive drug-taking: the role of the habenula in addiction. Neurosci Biobehav Rev. https://doi.org/10.1016/j.neubiorev.2018.06.018
Lee HW, Yang SH, Kim JY, Kim H (2019) The role of the medial habenula cholinergic system in addiction and emotion-associated behaviors. Front Psychiatry 10:14309–14318. https://doi.org/10.3389/fpsyt.2019.00100
Stewart JL, May AC, Aupperle RL, Bodurka J (2019) Forging neuroimaging targets for recovery in opioid use disorder. Front Psychiatry 10:760–815. https://doi.org/10.3389/fpsyt.2019.00117
Isometsä ET (2001) Psychological autopsy studies–a review. Eur Psychiatry J Assoc Eur Psychiatr 16:379–385
Bernstein HG, Stanarius A, Baumann B, Henning H, Krell D, Danos P, Falkai P, Bogerts B (1998) Nitric oxide synthase-containing neurons in the human hypothalamus: reduced number of immunoreactive cells in the paraventricular nucleus of depressive patients and schizophrenics. Neuroscience 83:867–875. https://doi.org/10.1016/s0306-4522(97)00461-2
Bernstein HG, Baumann B, Danos P, Diekmann S, Bogerts B, Gundelfinger ED, Braunewell KH (1999) Regional and cellular distribution of neural visinin-like protein immunoreactivities (VILIP-1 and VILIP-3) in human brain. J Neurocytol 28:655–662
Gundersen HJ, Jensen EB (1987) The efficiency of systematic sampling in stereology and its prediction. J Microsc 147:229–263
Díaz E, Bravo D, Rojas X, Concha ML (2011) Morphologic and immunohistochemical organization of the human habenular complex. J Comp Neurol 519:3727–3747. https://doi.org/10.1002/cne.22687
Baumann B, Danos P, Krell D, Diekmann S, Leschinger A, Stauch R, Wurthmann C, Bernstein HG, Bogerts B (1999) Reduced volume of limbic system-affiliated basal ganglia in mood disorders: preliminary data from a postmortem study. J Neuropsychiatry Clin Neuroscie 11:71–78
Carl M (2012) Habenula circuit development. Past Present Fut. https://doi.org/10.3389/fnins.2012.00051/abstract
Savitz JB, Nugent AC, Bogers W, Roiser JP, Bain EE, Neumeister A, Zarate CA, Manji HK, Cannon DM, Marrett S, Henn F, Charney DS, Drevets WC (2011) Habenula volume in bipolar disorder and major depressive disorder: a high-resolution magnetic resonance imaging study. Biol Psychiat 69:336–343. https://doi.org/10.1016/j.biopsych.2010.09.027
Savitz JB, Bonne O, Nugent AC, Vythilingam M, Bogers W, Charney DS, Drevets WC (2011) Habenula volume in post-traumatic stress disorder measured with high-resolution MRI. Biol Mood Anxiety Disord 1:7–5. https://doi.org/10.1186/2045-5380-1-7
Carceller-Sindreu M, de Diego-Adeliño J, Serra-Blasco M, Vives-Gilabert Y, n-Blanco AM, Puigdemont D, Álvarez E, Pérez V, Portella MJ (2015) Volumetric MRI study of the habenula in first episode, recurrent and chronic major depression. Eur Neuropsychopharmacol. https://doi.org/10.1016/j.euroneuro.2015.08.009
Schmidt FM, Schindler S, Adamidis M, Strauß M, Tränkner A, Trampel R, Walter M, Hegerl U, Turner R, Geyer S, Schönknecht P (2016) Habenula volume increases with disease severity in unmedicated major depressive disorder as revealed by 7T MRI. Eur Arch Psychiatry Clin Neurosci. https://doi.org/10.1007/s00406-016-0675-8
Luan SX, Zhang L, Wang R, Zhao H, Liu C (2019) A resting-state study of volumetric and functional connectivity of the habenular nucleus in treatment-resistant depression patients. Brain Behav 9:e01229–e1311. https://doi.org/10.1002/brb3.1229
Zhang L, Wang H, Luan S, Yang S, Wang Z, Wang J, Zhao H (2017) Altered volume and functional connectivity of the habenula in schizophrenia. Front Hum Neurosci 11:S1–9. https://doi.org/10.3389/fnhum.2017.00636
Li L, Lu G, Yao H, Zhhao Y, Feng Z, Yew DT (2005) Postmortem changes in the central nervous system and adrenal medulla of the heroin addicts. Int J Neurosci 115:1443–1449. https://doi.org/10.1080/00207450590956549
Cunha-Oliveira T, Rego AC, Garrido J, Borges F, Macedo T, Oliveira CR (2007) Street heroin induces mitochondrial dysfunction and apoptosis in rat cortical neurons. J Neurochem 101:543–554. https://doi.org/10.1111/j.1471-4159.2006.04406.x
Cunha-Oliveira T, Rego AC, Garrido J, Borges F, Macedo T, Oliveira CR (2010) Neurotoxicity of heroin–cocaine combinations in rat cortical neurons. Toxicology 276:11–17. https://doi.org/10.1016/j.tox.2010.06.009
Tan M, Li Z, Ma S, Luo J, Xu S, Lu A, Gan W, Su P, Lin H, Li S, Lai B (2013) Heroin activates Bim via c-Jun N-terminal kinase/c-Jun pathway to mediate neuronal apoptosis. Neuroscience 233:1–8. https://doi.org/10.1016/j.neuroscience.2012.12.005
Zhang J (2015) Heroin activates ATF3 and CytC via c-Jun N-terminal kinase pathways to mediate neuronal apoptosis. Med Sci Monit Basic Res 21:53–62. https://doi.org/10.12659/MSMBR.893827
Fakhoury M (2017) The habenula in psychiatric disorders: more than three decades of translational investigation. Neurosci Biobehav Rev. https://doi.org/10.1016/j.neubiorev.2017.02.010
Aizawa H, Zhu M (2019) Toward an understanding of the habenula’s various roles in human depression. Psychiatry Clin Neurosci 73:607–612. https://doi.org/10.1111/pcn.12892
Gold PW, Kadriu B (2019) A major role for the lateral habenula in depressive illness: physiologic and molecular mechanisms. Front Psychiatry 10:89–97. https://doi.org/10.3389/fpsyt.2019.00320
Velasquez KM, Molfese DL, Salas R (2014) The role of the habenula in drug addiction. Front Hum Neurosci 8:174. https://doi.org/10.3389/fnhum.2014.00174
Schmidt ERE, Pasterkamp RJ (2017) The molecular mechanisms controlling morphogenesis and wiring of the habenula. Pharmacol Biochem Behav 162:29–37. https://doi.org/10.1016/j.pbb.2017.08.008
Hétu S, Luo Y, Saez I, D'Ardenne K, Lohrenz T, Montague PR (2016) Asymmetry in functional connectivity of the human habenula revealed by high-resolution cardiac-gated resting state imaging. Hum Brain Mapp 37:2602–2615. https://doi.org/10.1002/hbm.23194
Andres KH, von Düring M, Veh RW (1999) Subnuclear organization of the rat habenular complexes. J Comp Neurol 407:130–150. https://doi.org/10.1002/(sici)1096-9861(19990428)407(1<130:aid-cne10>3.0.co;2-8)
Geisler S, Andres KH, Veh RDW (2003) Morphologic and cytochemical criteria for the identification and delineation of individual subnuclei within the lateral habenular complex of the rat. J Comp Neurol 458:78–97. https://doi.org/10.1002/cne.10566
Marburg O (1944) The structure and fiber connections of the human habenula. J Comp Neurol 80:211–233. https://doi.org/10.1002/cne.900800205
Batalla A, Homberg JR, Lipina TV, Sescousse G, Luijten M, Ivanova SA, Schellekens AFA, Loonen AJM (2017) The role of the habenula in the transition from reward to misery in substance use and mood disorders. Neurosci Biobehav Rev 80:276–285. https://doi.org/10.1016/j.neubiorev.2017.03.019
Matsumoto M, Hikosaka O (2007) Lateral habenula as a source of negative reward signals in dopamine neurons. Nature 447:1111–1115. https://doi.org/10.1038/nature05860
Ellison G (2002) Neural degeneration following chronic stimulant abuse reveals a weak link in brain, fasciculus retroflexus, implying the loss of forebrain control circuitry. Eur Neuropsychopharmacol 12:287–297. https://doi.org/10.1016/s0924-977x(02)00020-2
Ellison G (1992) Continuous amphetamine and cocaine have similar neurotoxic effects in lateral habenular nucleus and fasciculus retroflexus. Brain Res 598:353–356. https://doi.org/10.1016/0006-8993(92)90207-p
Lax E, Friedman A, Croitoro O, Sudai E, Ben-Moshe H, Redlus L, Sasson E, Katzir T, Assaf Y, Yadid G (2013) Neurodegeneration of lateral habenula efferent fibers after intermittent cocaine administration: Implications for deep brain stimulation. Neuropharmacology. https://doi.org/10.1016/j.neuropharm.2013.06.034
Acknowledgements
The Magdeburg Brain Collection and subsequently this investigation have been supported in part by the Stanley Medical Research Foundation (Grant No. 07R-1832) to BB and JS, the Saxony-Anhalt Ministry of Research (XN3594O/0405M, N2-OVGU), the German Ministry of Research („BrainNet“, BMBF NBL-3/2 and 01ZZ0407) and the Alfried-Krupp-von-Bohlen-und-Halbach foundation. We are grateful to KB, BJ and GM-L for their excellent technical assistance.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflicts of interest
All authors declare that they have no conflicts of interest.
Ethical approval
Sampling and preservation of the human brain material were done in accordance with the Declaration of Helsinki, German Law and approval by the local institutional review board.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Müller, U.J., Ahrens, M., Vasilevska, V. et al. Reduced habenular volumes and neuron numbers in male heroin addicts: a post-mortem study. Eur Arch Psychiatry Clin Neurosci 271, 835–845 (2021). https://doi.org/10.1007/s00406-020-01195-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00406-020-01195-y