Abstract
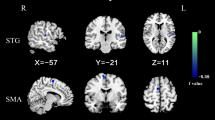
Abnormalities of the cerebellum and default-mode network (DMN) in patients with obsessive–compulsive disorder (OCD) have been widely reported. However, alterations of reciprocal functional connections between the cerebellum and DMN at rest in OCD remain unclear. Forty patients with OCD and 38 gender-, age-, and education-matched healthy controls (HCs) underwent resting-state functional magnetic resonance imaging scan. Seed-based functional connectivity (FC) and support vector machine (SVM) were applied to analyze the imaging data. Compared with HCs, patients with OCD exhibited increased FCs between the left Crus I–left superior medial prefrontal cortex (MPFC) and between the right Crus I–left superior MPFC, left middle MPFC, and left middle temporal gyrus (MTG). A significantly negative correlation was observed between the right Crus I–left MTG connectivity and the Yale–Brown Obsessive–Compulsive Scale compulsion subscale scores in the OCD group (r = − 0.476, p = 0.002, Bonferroni corrected). SVM classification analysis indicated that a combination of the left Crus I–left superior MPFC connectivity and the right Crus I–left middle MPFC connectivity can be used to discriminate patients with OCD from HCs with a sensitivity of 85.00%, specificity of 68.42%, and accuracy of 76.92%. Our study highlights the contribution of the cerebellar–DMN connectivity in OCD pathophysiology and provides new findings to OCD research.





Similar content being viewed by others
References
Ruscio AM, Stein DJ, Chiu WT, Kessler RC (2010) The epidemiology of obsessive–compulsive disorder in the National Comorbidity Survey Replication. Mol Psychiatry 15(1):53–63. https://doi.org/10.1038/mp.2008.94
Figee M, Wielaard I, Mazaheri A, Denys D (2013) Neurosurgical targets for compulsivity: what can we learn from acquired brain lesions? Neurosci Biobehav Rev 37(3):328–339. https://doi.org/10.1016/j.neubiorev.2013.01.005
Schmahmann JD, Weilburg JB, Sherman JC (2007) The neuropsychiatry of the cerebellum-insights from the clinic. Cerebellum 6(3):254–267. https://doi.org/10.1080/14734220701490995
Stoodley CJ (2012) The cerebellum and cognition: evidence from functional imaging studies. Cerebellum 11(2):352–365. https://doi.org/10.1007/s12311-011-0260-7
de Wit SJ, Alonso P, Schweren L, Mataix-Cols D, Lochner C, Menchon JM, Stein DJ, Fouche JP, Soriano-Mas C, Sato JR, Hoexter MQ, Denys D, Nakamae T, Nishida S, Kwon JS, Jang JH, Busatto GF, Cardoner N, Cath DC, Fukui K, Jung WH, Kim SN, Miguel EC, Narumoto J, Phillips ML, Pujol J, Remijnse PL, Sakai Y, Shin NY, Yamada K, Veltman DJ, van den Heuvel OA (2014) Multicenter voxel-based morphometry mega-analysis of structural brain scans in obsessive–compulsive disorder. Am J Psychiatry 171(3):340–349. https://doi.org/10.1176/appi.ajp.2013.13040574
Hu X, Du M, Chen L, Li L, Zhou M, Zhang L, Liu Q, Lu L, Mreedha K, Huang X, Gong Q (2017) Meta-analytic investigations of common and distinct grey matter alterations in youths and adults with obsessive–compulsive disorder. Neurosci Biobehav Rev 78:91–103. https://doi.org/10.1016/j.neubiorev.2017.04.012
Pujol J, Soriano-Mas C, Alonso P, Cardoner N, Menchón JM, Deus J, Vallejo J (2004) Mapping structural brain alterations in obsessive–compulsive disorder. Arch Gen Psychiatry 61(7):720–730. https://doi.org/10.1001/archpsyc.61.7.720
Kim JJ, Lee MC, Kim J, Kim IY, Kim SI, Han MH, Chang KH, Kwon JS (2001) Grey matter abnormalities in obsessive–compulsive disorder: statistical parametric mapping of segmented magnetic resonance images. Brit J Psychiat 179:330–334. https://doi.org/10.1192/bjp.179.4.330
Narayanaswamy JC, Jose D, Kalmady SV, Agarwal SM, Venkatasubramanian G, Janardhan Reddy YC (2016) Cerebellar volume deficits in medication-naive obsessive compulsive disorder. Psychiatry Res Neuroimaging 254:164–168. https://doi.org/10.1016/j.pscychresns.2016.07.005
Ping L, Su-Fang L, Hai-Ying H, Zhang-Ye D, Jia L, Zhi-Hua G, Hong-Fang X, Yu-Feng Z, Zhan-Jiang L (2013) Abnormal spontaneous neural activity in obsessive–compulsive disorder: a resting-state functional magnetic resonance imaging study. PLoS ONE 8(6):e67262. https://doi.org/10.1371/journal.pone.0067262
Qiu L, Fu X, Wang S, Tang Q, Chen X, Cheng L, Zhang F, Zhou Z, Tian L (2017) Abnormal regional spontaneous neuronal activity associated with symptom severity in treatment-naive patients with obsessive–compulsive disorder revealed by resting-state functional MRI. Neurosci Lett 640:99–104. https://doi.org/10.1016/j.neulet.2017.01.024
Meng Z, Zhang Z, Fan Q, Li Y (2018) Altered fractional amplitude of low frequency fluctuations in unmedicated female patients with obsessive–compulsive disorder. Conf Proc IEEE Eng Med Biol Soc 2018:1144–1147. https://doi.org/10.1109/EMBC.2018.8512490
Hou JM, Wu WJ, Lin Y, Wang J, Zhou DQ, Guo JW, Gu SS, He M, Ahmed S, Hu JN, Qu W, Li HT (2012) Localization of cerebral functional deficits in patients with obsessive–compulsive disorder: a resting-state fMRI study. J Affect Disord 138(3):313–321. https://doi.org/10.1016/j.jad.2012.01.022
Zhong Z, Yang X, Cao R, Li P, Li Z, Lv L, Zhang D (2019) Abnormalities of white matter microstructure in unmedicated patients with obsessive–compulsive disorder: changes after cognitive behavioral therapy. Brain Behav 9(2):e01201. https://doi.org/10.1002/brb3.1201
Sanematsu H, Nakao T, Yoshiura T, Nabeyama M, Togao O, Tomita M, Masuda Y, Nakatani E, Nakagawa A, Kanba S (2010) Predictors of treatment response to fluvoxamine in obsessive–compulsive disorder: an fMRI study. J Psychiatr Res 44(4):193–200. https://doi.org/10.1016/j.jpsychires.2009.08.007
Ding Yudan, Yangpan Ou, Pan Pan, Shan Xiaoxiao, Chen Jindong, Liu Feng, Zhao Jingping, Guo W (2019) Cerebellar structural and functional abnormalities in first-episode and drug-naive patients with schizophrenia: a meta-analysis. Psychiatry Res Neuroimaging 283(2019):24–33. https://doi.org/10.1016/j.pscychresns.2018.11.009
Buckner RL, Krienen FM, Castellanos A, Diaz JC, Yeo BTT (2011) The organization of the human cerebellum estimated by intrinsic functional connectivity. J Neurophysiol 106(5):2322–2345. https://doi.org/10.1152/jn.00339.2011
O’Reilly JX, Beckmann CF, Tomassini V, Ramnani N, Johansen-Berg H (2010) Distinct and overlapping functional zones in the cerebellum defined by resting state functional connectivity. Cereb Cortex 20(4):953–965. https://doi.org/10.1093/cercor/bhp157
Andrews-Hanna JR, Reidler JS, Sepulcre J, Poulin R, Buckner RL (2010) Functional-anatomic fractionation of the brain’s default network. Neuron 65(4):550–562. https://doi.org/10.1016/j.neuron.2010.02.005
Beucke JC, Sepulcre J, Eldaief MC, Sebold M, Kathmann N, Kaufmann C (2014) Default mode network subsystem alterations in obsessive–compulsive disorder. Br J Psychiatry 205(5):376–382. https://doi.org/10.1192/bjp.bp.113.137380
Buckner RL, Andrews-Hanna JR, Schacter DL (2008) The brain’s default network: anatomy, function, and relevance to disease. Ann N Y Acad Sci 1124:1–38. https://doi.org/10.1196/annals.1440.011
Guo W, Liu F, Liu J, Yu M, Zhang Z, Liu G, Xiao C, Zhao J (2015) Increased cerebellar-default-mode-network connectivity in drug-naive major depressive disorder at rest. Medicine 94(9):e560. https://doi.org/10.1097/md.0000000000000560
Wang H, Guo W, Liu F, Chen J, Wu R, Zhang Z, Yu M, Li L, Zhao J (2016) Clinical significance of increased cerebellar default-mode network connectivity in resting-state patients with drug-naive somatization disorder. Medicine 95(28):e4043. https://doi.org/10.1097/md.0000000000004043
Fan J, Zhong M, Gan J, Liu W, Niu C, Liao H, Zhang H, Yi J, Chan R, Tan C (2017) Altered connectivity within and between the default mode, central executive, and salience networks in obsessive–compulsive disorder. J Affect Disord 223:106–114. https://doi.org/10.1016/j.jad.2017.07.041
Hou J, Song L, Zhang W, Wu W, Wang J, Zhou D, Qu W, Guo J, Gu S, He M (2013) Morphologic and functional connectivity alterations of corticostriatal and default mode network in treatment-naïve patients with obsessive–compulsive disorder. PLoS ONE 8(12):e83931. https://doi.org/10.1371/journal.pone.0083931
Koçak OM, Kale E, Çiçek M (2012) Default mode network connectivity differences in obsessive–compulsive disorder. Act Nerv Super 54(3–4):118–124. https://doi.org/10.1007/bf03379589
Koch K, Reess TJ, Rus OG, Gursel DA, Wagner G, Berberich G, Zimmer C (2018) Increased default mode network connectivity in obsessive–compulsive disorder during reward processing. Front Psychiatry 9:254. https://doi.org/10.3389/fpsyt.2018.00254
Peng ZW, Xu T, He QH, Shi CZ, Wei Z, Miao GD, Jing J, Lim KO, Zuo XN, Chan RC (2014) Default network connectivity as a vulnerability marker for obsessive compulsive disorder. Psychol Med 44(7):1475–1484. https://doi.org/10.1017/S0033291713002250
Posner J, Song I, Lee S, Rodriguez CI, Moore H, Marsh R, Blair SH (2017) Increased functional connectivity between the default mode and salience networks in unmedicated adults with obsessive–compulsive disorder. Hum Brain Mapp 38:678–687. https://doi.org/10.1002/hbm.23408
Chen Y, Ou Y, Lv D, Yang R, Li S, Jia C, Wang Y, Meng X, Cui H, Li C, Sun Z, Wang X, Guo W, Li P (2019) Altered network homogeneity of the default-mode network in drug-naive obsessive–compulsive disorder. Prog Neuropsychopharmacol Biol Psychiatry 93:77–83. https://doi.org/10.1016/j.pnpbp.2019.03.008
Xu T, Zhao Q, Wang P, Fan Q, Chen J, Zhang H, Yang Z, Stein DJ, Wang Z (2018) Altered resting-state cerebellar-cerebral functional connectivity in obsessive–compulsive disorder. Psychol Med:1-10. https://doi.org/10.1017/s0033291718001915
First MB, Spitzer RL, Gibbon M, JBW W (1996) Structured clinical interview for DSM-IV Axis I disorders. American Psychiatric Press, Clinician Version (SCID-CV)
Yan CG, Wang XD, Zuo XN, Zang YF (2016) DPABI: data processing & analysis for (resting-state) brain Imaging. Neuroinformatics 14(3):339–351. https://doi.org/10.1007/s12021-016-9299-4
Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE (2012) Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. neuroimage 59 (3):2142-2154. https://doi.org/10.1016/j.neuroimage.2011.10.018
Yan CG, Cheung B, Kelly C, Colcombe S, Craddock RC, Martino AD, Li Q, Zuo XN, Castellanos FX, Milham MP (2013) A comprehensive assessment of regional variation in the impact of head micromovements on functional connectomics. neuroimage 76 (1):183-201. https://doi.org/10.1016/j.neuroimage.2013.03.004
Hahamy A, Calhoun V, Pearlson G, Harel M, Stern N, Attar F, Malach R, Salomon R (2014) Save the global: global signal connectivity as a tool for studying clinical populations with functional magnetic resonance imaging. Brain Connect 4(6):395–403. https://doi.org/10.1089/brain.2014.0244
Christophe H, Nirav K, Daniel N, Katherine P, Beckmann CF, Vinod M, Greicius MD (2009) Distinct cerebellar contributions to intrinsic connectivity networks. J Neurosci 47(26):8586–8594. https://doi.org/10.1523/JNEUROSCI.1868-09.2009
Wang L, Zou F, Shao Y, Ye E, Jin X, Tan S, Hu D, Yang Z (2014) Disruptive changes of cerebellar functional connectivity with the default mode network in schizophrenia. Schizophr Res 160(1–3):67–72. https://doi.org/10.1016/j.schres.2014.09.034
Guo W, Liu F, Zhang Z, Liu G, Liu J, Yu L, Xiao C, Zhao J (2015) Increased cerebellar functional connectivity with the default-mode network in unaffected siblings of schizophrenia patients at rest. Schizophr Bull 41(6):1317–1325. https://doi.org/10.1093/schbul/sbv062
Krienen FM, Buckner RL (2009) Segregated fronto-cerebellar circuits revealed by intrinsic functional connectivity. Cereb Cortex 19(10):2485–2497. https://doi.org/10.1093/cercor/bhp135
Song XW, Dong ZY, Long XY, Li SF, Zuo XN, Zhu CZ, He Y, Yan CG, Zang YF (2011) REST: a toolkit for resting-state functional magnetic resonance imaging data processing. PLoS ONE 6(9):e25031. https://doi.org/10.1371/journal.pone.0025031
Anticevic A, Hu S, Zhang S, Savic A, Billingslea E, Wasylink S, Repovs G, Cole MW, Bednarski S, Krystal JH, Bloch MH, Li CS, Pittenger C (2014) Global resting-state functional magnetic resonance imaging analysis identifies frontal cortex, striatal, and cerebellar dysconnectivity in obsessive–compulsive disorder. Biol Psychiatry 75(8):595–605. https://doi.org/10.1016/j.biopsych.2013.10.021
Fan J, Zhong M, Gan J, Liu W, Niu C, Liao H, Zhang H, Tan C, Yi J, Zhu X (2017) Spontaneous neural activity in the right superior temporal gyrus and left middle temporal gyrus is associated with insight level in obsessive–compulsive disorder. J Affect Disord 207:203–211. https://doi.org/10.1016/j.jad.2016.08.027
Saxena S, Brody AL, Schwartz JM, Baxter LR (1998) Neuroimaging and frontal-subcortical circuitry in obsessive–compulsive disorder. The British journal of psychiatry Supplement 35:26–37. https://doi.org/10.1016/S0006-3223(97)90372-3
Alalade E, Denny K, Potter G, Steffens D, Wang L (2011) Altered cerebellar-cerebral functional connectivity in geriatric depression. PLoS ONE 6(5):e20035. https://doi.org/10.1371/journal.pone.0020035
Radua J, Mataixcols D (2009) Voxel-wise meta-analysis of grey matter changes in obsessive–compulsive disorder. Br J Psychiatry 195(5):393–402. https://doi.org/10.1192/bjp.bp.108.055046
Gürsel DA, Avram M, Sorg C, Brandl F, Koch K (2018) Frontoparietal areas link impairments of large-scale intrinsic brain networks with aberrant fronto-striatal interactions in OCD: a meta-analysis of resting-state functional connectivity. Neurosci Biobehav Rev 87:151–160. https://doi.org/10.1016/j.neubiorev.2018.01.016
Apergis-Schoute AM, Gillan CM, Fineberg NA, Fernandez-Egea E, Sahakian BJ, Robbins TW (2017) Neural basis of impaired safety signaling in obsessive compulsive disorder. Proc Natl Acad Sci U S A 114(12):3216–3221. https://doi.org/10.1073/pnas.1609194114
Manning EE, Dombrovski AY, Torregrossa MM (2019) Ahmari SE (2018) Impaired instrumental reversal learning is associated with increased medial prefrontal cortex activity in Sapap3 knockout mouse model of compulsive behavior. Neuropsychopharmacology. https://doi.org/10.1038/s41386-018-0307-2
An SK, Mataix-Cols D, Lawrence NS, Wooderson S, Giampietro V, Speckens A, Brammer MJ, Phillips ML (2009) To discard or not to discard: the neural basis of hoarding symptoms in obsessive–compulsive disorder. Mol Psychiatr 14(3):318–331. https://doi.org/10.1038/sj.mp.4002129
Desmond JE, Fiez JA (1998) Neuroimaging studies of the cerebellum: language, learning and memory. Trends in cognitive sciences 2(9):355–362. https://doi.org/10.1016/S1364-6613(98)01211-X
Collette F, Van der Linden M, Laureys S, Arigoni F, Delfiore G, Degueldre C, Luxen A, Salmon E (2007) Mapping the updating process: common and specific brain activations across different versions of the running span task. Cortex 43(1):146–158. https://doi.org/10.1016/s0010-9452(08)70452-0
Bu X, Hu X, Zhang L, Li B, Zhou M, Lu L, Hu X, Li H, Yang Y, Tang W, Gong Q, Huang X (2019) Investigating the predictive value of different resting-state functional MRI parameters in obsessive–compulsive disorder. Transl Psychiatry 9(1):17. https://doi.org/10.1038/s41398-018-0362-9
Fox MD, Corbetta M, Snyder AZ, Vincent JL, Raichle ME (2006) Spontaneous neuronal activity distinguishes human dorsal and ventral attention systems. Proc Natl Acad Sci U S A 103(26):10046–10051. https://doi.org/10.1073/pnas.0604187103
Hou JM, Zhao M, Zhang W, Song LH, Wu WJ, Wang J, Zhou DQ, Xie B, He M, Guo JW, Qu W, Li HT (2014) Resting-state functional connectivity abnormalities in patients with obsessive–compulsive disorder and their healthy first-degree relatives. J Psychiatr Neurosci 39(5):304–311. https://doi.org/10.1503/Jpn.130220
Chen SH, Desmond JE (2005) Cerebrocerebellar networks during articulatory rehearsal and verbal working memory tasks. NeuroImage 24(2):332–338. https://doi.org/10.1016/j.neuroimage.2004.08.032
Acknowledgements
We thank all participants who willingly gave their time to provide data.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Ethical standards
This study was approved by the Research Ethics Committee of Qiqihar Medical University, China. All participants were informed regarding the study procedures and signed a written informed consent.
Financial disclosures
This study was supported by grants from Heilongjiang Natural Science Foundation of China (LH2019H064).
Additional information
Dan Lv and Yangpan Ou contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lv, D., Ou, Y., Chen, Y. et al. Increased cerebellar–default-mode network connectivity at rest in obsessive–compulsive disorder. Eur Arch Psychiatry Clin Neurosci 270, 1015–1024 (2020). https://doi.org/10.1007/s00406-019-01070-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00406-019-01070-5