Abstract
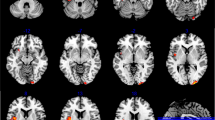
Intermediate phenotype could be used to investigate genetic susceptibility. However, genetic and environmental heterogeneity may interfere with identification of intermediate phenotypes. In this study, we minimized these interferences by using a novel group strategy. A total of 22 drug-naive and first-episode schizophrenia (FES) patients, along with 22 of their kin healthy siblings (HS), 22 non-kin healthy siblings (nHS) of other schizophrenia patients and 22 healthy controls (HC), were recruited. Brain imaging was acquired from the participants. Voxel-based analysis was used to investigate differences in white matter integrity derived from diffusion tensor imaging among the four groups. Two cognitive tests related to our findings were selected to confirm the related phenotypic changes. All of the FES, HS, and nHS groups showed decreased fractional anisotropy (FA) values in the left inferior frontal gyrus (IFG) compared with the HC group (p < 0.05, FDR corrected). The scores of Hopkins Verbal learning Test-Revised and Animal Naming in FES patients were significantly lower than in participants belonging to the other three groups (p < 0.05). Significant correlation between Animal Naming scores and FA values in the left IFG was found in FES patients (r = 0.53, p = 0.01). Moreover, FES patients also showed decreased FA values in the left medial frontal gyrus, left inferior temporal gyrus, left parahippocampal gyrus, left posterior cingulate, and right middle temporal gyrus compared with HC (p < 0.05, FDR corrected). Decreased FA values in the left IFG is a possible intermediate phenotype of schizophrenia, and this finding supports the hypothesis that disrupted connectivity of white matter may be the key substrate of schizophrenia.




Similar content being viewed by others
References
Kleinman JE, Law AJ, Lipska BK, Hyde TM, Ellis JK, Harrison PJ, Weinberger DR (2011) Genetic neuropathology of schizophrenia: new approaches to an old question and new uses for postmortem human brains. Biol Psychiatry 69(2):140–145. doi:10.1016/j.biopsych.2010.10.032
O’Donovan MC, Craddock N, Norton N, Williams H, Peirce T, Moskvina V, Nikolov I, Hamshere M, Carroll L, Georgieva L, Dwyer S, Holmans P, Marchini JL, Spencer CC, Howie B, Leung HT, Hartmann AM, Moller HJ, Morris DW, Shi Y, Feng G, Hoffmann P, Propping P, Vasilescu C, Maier W, Rietschel M, Zammit S, Schumacher J, Quinn EM, Schulze TG, Williams NM, Giegling I, Iwata N, Ikeda M, Darvasi A, Shifman S, He L, Duan J, Sanders AR, Levinson DF, Gejman PV, Cichon S, Nothen MM, Gill M, Corvin A, Rujescu D, Kirov G, Owen MJ, Buccola NG, Mowry BJ, Freedman R, Amin F, Black DW, Silverman JM, Byerley WF, Cloninger CR, Molecular Genetics of Schizophrenia C (2008) Identification of loci associated with schizophrenia by genome-wide association and follow-up. Nat Genet 40(9):1053–1055. doi:10.1038/ng.201
Neddens J, Bagorda F, Busche A, Horstmann S, Moll G, Dawirs R, Teuchert-Noodt G (2003) Epigenetic factors differentially influence postnatal maturation of serotonin (5-HT) innervation in cerebral cortex of gerbils: interaction of rearing conditions and early methamphetamine challenge. Dev Brain Res 146(1):119–130
Tsuang MT, Faraone SV, Lyons MJ (1993) Identification of the phenotype in psychiatric genetics. Eur Arch Psychiatry Clin Neurosci 243(3–4):131–142
Rasetti R, Weinberger DR (2011) Intermediate phenotypes in psychiatric disorders. Curr Opin Genet Dev 21(3):340–348. doi:10.1016/j.gde.2011.02.003
Lenzenweger MF (2013) Endophenotype, intermediate phenotype, biomarker: definitions, concept comparisons, clarifications. Depress Anxiety 30(3):185–189. doi:10.1002/da.22042
Buchsbaum MS, Friedman J, Buchsbaum BR, Chu KW, Hazlett EA, Newmark R, Schneiderman JS, Torosjan Y, Tang C, Hof PR, Stewart D, Davis KL, Gorman J (2006) Diffusion tensor imaging in schizophrenia. Biol Psychiatry 60(11):1181–1187. doi:10.1016/j.biopsych.2005.11.028
Kyriakopoulos M, Vyas NS, Barker GJ, Chitnis XA, Frangou S (2008) A diffusion tensor imaging study of white matter in early-onset schizophrenia. Biol Psychiatry 63(5):519–523. doi:10.1016/j.biopsych.2007.05.021
Shepherd AM, Laurens KR, Matheson SL, Carr VJ, Green MJ (2012) Systematic meta-review and quality assessment of the structural brain alterations in schizophrenia. Neurosci Biobehav Rev 36(4):1342–1356. doi:10.1016/j.neubiorev.2011.12.015
Nenadic I, Gaser C, Sauer H (2012) Heterogeneity of brain structural variation and the structural imaging endophenotypes in schizophrenia. Neuropsychobiology 66(1):44–49. doi:10.1159/000338547
American Psychiatric Association (2000) Diagnostic and statistical manual of mental disorders, 4th edn. American Psychiatric Publishing, Washingtons
First MB, Spitzer RL, Gibbon M, Williams JB (1997) Structured clinical interview for DSM-IV axis I disorders, clinician version (SCID-CV). American Psychiatric Association, Washington
Thomason ME, Thompson PM (2011) Diffusion imaging, white matter, and psychopathology. Annu Rev Clin Psychol 7:63–85
Quan M, Lee S-H, Kubicki M, Kikinis Z, Rathi Y, Seidman LJ, Mesholam-Gately RI, Goldstein JM, McCarley RW, Shenton ME, Levitt JJ (2013) White matter tract abnormalities between rostral middle frontal gyrus, inferior frontal gyrus and striatum in first-episode schizophrenia. Schizophr Res 145(1-3):1–10
Jeong B, Wible CG, Hashimoto RI, Kubicki M (2009) Functional and anatomical connectivity abnormalities in left inferior frontal gyrus in schizophrenia. Hum Brain Mapp 30(12):4138–4151
Kuroki N, Shenton ME, Salisbury DF, Hirayasu Y, Onitsuka T, Ersner-Hershfield H, Yurgelun-Todd D, Kikinis R, Jolesz FA, McCarley RW (2006) Middle and inferior temporal gyrus gray matter volume abnormalities in first-episode schizophrenia: an MRI study. Am J Psychiatry 163(12):2103–2110. doi:10.1176/appi.ajp.163.12.2103
Honea RA, Meyer-Lindenberg A, Hobbs KB, Pezawas L, Mattay VS, Egan MF, Verchinski B, Passingham RE, Weinberger DR, Callicott JH (2008) Is gray matter volume an intermediate phenotype for schizophrenia? A voxel-based morphometry study of patients with schizophrenia and their healthy siblings. Biol Psychiatry 63(5):465–474
Eyler LT, Jeste DV, Brown GG (2008) Brain response abnormalities during verbal learning among patients with schizophrenia. Psychiatry Res 162(1):11–25. doi:10.1016/j.pscychresns.2007.03.009
Bonner-Jackson A, Haut K, Csernansky JG, Barch DM (2005) The influence of encoding strategy on episodic memory and cortical activity in schizophrenia. Biol Psychiatry 58(1):47–55. doi:10.1016/j.biopsych.2005.05.011
Winchester CL, Ohzeki H, Vouyiouklis DA, Thompson R, Penninger JM, Yamagami K, Norrie JD, Hunter R, Pratt JA, Morris BJ (2012) Converging evidence that sequence variations in the novel candidate gene MAP2K7 (MKK7) are functionally associated with schizophrenia. Hum Mol Genet 21(22):4910–4921. doi:10.1093/hmg/dds331
Ohi K, Hashimoto R, Yasuda Y, Kiribayashi M, Iike N, Yoshida T, Azechi M, Ikezawa K, Takahashi H, Morihara T, Ishii R, Tagami S, Iwase M, Okochi M, Kamino K, Kazui H, Tanaka T, Kudo T, Takeda M (2009) TATA box-binding protein gene is associated with risk for schizophrenia, age at onset and prefrontal function. Genes Brain Behav 8(4):473–480. doi:10.1111/j.1601-183X.2009.00497.x
Kimoto S, Bazmi HH, Lewis DA (2014) Lower expression of glutamic acid decarboxylase 67 in the prefrontal cortex in schizophrenia: contribution of altered regulation by Zif268. Am J Psychiatry 171(9):969–978. doi:10.1176/appi.ajp.2014.14010004
Rosen H, Petersen S, Linenweber M, Snyder A, White D, Chapman L, Dromerick A, Fiez J, Corbetta M (2000) Neural correlates of recovery from aphasia after damage to left inferior frontal cortex. Neurology 55(12):1883–1894
Wirth M, Jann K, Dierks T, Federspiel A, Wiest R, Horn H (2011) Semantic memory involvement in the default mode network: a functional neuroimaging study using independent component analysis. Neuroimage 54(4):3057–3066
Devlin JT, Matthews PM, Rushworth MF (2003) Semantic processing in the left inferior prefrontal cortex: a combined functional magnetic resonance imaging and transcranial magnetic stimulation study. J Cogn Neurosci 15(1):71–84
Heydebrand G, Weiser M, Rabinowitz J, Hoff AL, DeLisi LE, Csernansky JG (2004) Correlates of cognitive deficits in first episode schizophrenia. Schizophr Res 68(1):1–9. doi:10.1016/S0920-9964(03)00097-5
Hill SK, Beers SR, Kmiec JA, Keshavan MS, Sweeney JA (2004) Impairment of verbal memory and learning in antipsychotic-naive patients with first-episode schizophrenia. Schizophr Res 68(2–3):127–136. doi:10.1016/S0920-9964(03)00125-7
Cannon TD, Zorrilla LE, Shtasel D, Gur RE, Gur RC, Marco EJ, Moberg P, Price RA (1994) Neuropsychological functioning in siblings discordant for schizophrenia and healthy volunteers. Arch Gen Psychiatry 51(8):651–661
Hu M, Chen J, Li L, Zheng Y, Wang J, Guo X, Wu R, Zhao J (2011) Semantic fluency and executive functions as candidate endophenotypes for the early diagnosis of schizophrenia in Han Chinese. Neurosci Lett 502(3):173–177. doi:10.1016/j.neulet.2011.07.037
White T, Kendi AT, Lehericy S, Kendi M, Karatekin C, Guimaraes A, Davenport N, Schulz SC, Lim KO (2007) Disruption of hippocampal connectivity in children and adolescents with schizophrenia–a voxel-based diffusion tensor imaging study. Schizophr Res 90(1–3):302–307. doi:10.1016/j.schres.2006.09.032
Lyu HL, Hu MR, Eyler LT, Jin H, Wang J, Ou JJ, Guo XF, He Z, Liu F, Zhao JP, Guo WB (2015) Regional white matter abnormalities in drug-naive, first-episode schizophrenia patients and their healthy unaffected siblings. Aust NZ J Psychiatry 49(3):246–254. doi:10.1177/0004867414554268
Fujiwara H, Namiki C, Hirao K, Miyata J, Shimizu M, Fukuyama H, Sawamoto N, Hayashi T, Murai T (2007) Anterior and posterior cingulum abnormalities and their association with psychopathology in schizophrenia: a diffusion tensor imaging study. Schizophr Res 95(1–3):215–222
Newell KA, Zavitsanou K, Jew SK, Huang XF (2007) Alterations of muscarinic and GABA receptor binding in the posterior cingulate cortex in schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry 31(1):225–233
Ellison-Wright I, Bullmore E (2009) Meta-analysis of diffusion tensor imaging studies in schizophrenia. Schizophr Res 108(1):3–10
Hao YH, Liu ZN, Jiang TZ, Gong GL, Liu HH, Tan LH, Kuang F, Xu L, Yi YH, Zhang ZS (2006) White matter integrity of the whole brain is disrupted in first-episode schizophrenia. NeuroReport 17(1):23–26. doi:10.1097/01.wnr.0000195664.15090.46
Hao Y, Yan Q, Liu H, Xu L, Xue Z, Song X, Kaneko Y, Jiang T, Liu Z, Shan B (2009) Schizophrenia patients and their healthy siblings share disruption of white matter integrity in the left prefrontal cortex and the hippocampus but not the anterior cingulate cortex. Schizophr Res 114(1–3):128–135
Tang J, Liao Y, Zhou B, Tan C, Liu T, Hao W, Hu D, Chen X (2010) Abnormal anterior cingulum integrity in first episode, early-onset schizophrenia: a diffusion tensor imaging study. Brain Res 1343:199–205. doi:10.1016/j.brainres.2010.04.083
Acknowledgements
This research was supported by National R&D Special Fund for Health Profession by the Ministry of Health of the Peoples’ Republic of China (Grant No. 201002003), National Natural Science Foundation of China (81071093, 81270019, 81260210, 81361120396, 81360211, 81471363 and 30900483), and the Natural Science Foundations of Guangxi (2013GXNSFAA019107).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Jianjun Ou, Hailong Lyu, and Maorong Hu have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Ou, J., Lyu, H., Hu, M. et al. Decreased white matter FA values in the left inferior frontal gyrus is a possible intermediate phenotype of schizophrenia: evidences from a novel group strategy. Eur Arch Psychiatry Clin Neurosci 268, 89–98 (2018). https://doi.org/10.1007/s00406-016-0752-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00406-016-0752-z