Abstract
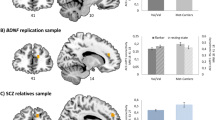
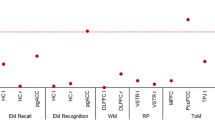
The alpha 1C subunit of the L-type voltage-gated calcium channel (CACNA1C) gene is one of the best replicated susceptibility loci for bipolar disorder, schizophrenia and major depression. It is involved in learning, memory and brain plasticity. Genetic studies using functional magnetic resonance imaging (fMRI) reported evidence of association with the CACNA1C single nucleotide polymorphism rs1006737 with functional correlates of episodic memory encoding and retrieval, especially activations in the hippocampus. These results, however, are inconsistent with regard to the magnitude and directionality of effect. In the present study, brain activation was measured with fMRI during an episodic memory encoding and retrieval task using neutral faces in two independent samples of 94 and 111 healthy subjects, respectively. Within whole brain analyses, a main effect of genotype emerged mainly in the right hippocampus during encoding as well as retrieval within the first sample: Carriers of the minor allele (A) exhibited lower activations compared to G/G allele carriers. This effect could be replicated within the second sample, however, only for the retrieval condition. The results strengthen findings that rs1006737 is associated with neural systems related to memory processes in hippocampal regions which are detectable in healthy subjects.

Similar content being viewed by others
References
Bedenbender J, Paulus FM, Krach S, Pyka M, Sommer J, Krug A, Witt SH, Rietschel M, Laneri D, Kircher T, Jansen A (2011) Functional connectivity analyses in imaging genetics: considerations on methods and data interpretation. PLoS ONE 6:e26354
Bigos KL, Mattay VS, Callicott JH, Straub RE, Vakkalanka R, Kolachana B, Hyde TM, Lipska BK, Kleinman JE, Weinberger DR (2010) Genetic variation in CACNA1C affects brain circuitries related to mental illness. Arch Gen Psychiatry 67:939–945
Blumenfeld RS, Ranganath C (2007) Prefrontal cortex and long-term memory encoding: an integrative review of findings from neuropsychology and neuroimaging. Neuroscientist 13:280–291
Bonner-Jackson A, Haut K, Csernansky JG, Barch DM (2005) The influence of encoding strategy on episodic memory and cortical activity in schizophrenia. Biol Psychiatry 58:47–55
Brassen S, Weber-Fahr W, Sommer T, Lehmbeck JT, Braus DF (2006) Hippocampal-prefrontal encoding activation predicts whether words can be successfully recalled or only recognized. Behav Brain Res 171:271–278
Braver TS, Barch DM, Kelley WM, Buckner RL, Cohen NJ, Miezin FM, Snyder AZ, Ollinger JM, Akbudak E, Conturo TE, Petersen SE (2001) Direct comparison of prefrontal cortex regions engaged by working and long-term memory tasks. Neuroimage 14:48–59
Erk S, Meyer-Lindenberg A, Schnell K, Opitz von Boberfeld C, Esslinger C, Kirsch P, Grimm O, Arnold C, Haddad L, Witt SH, Cichon S, Nothen MM, Rietschel M, Walter H (2010) Brain function in carriers of a genome-wide supported bipolar disorder variant. Arch Gen Psychiatry 67:803–811
Fernandez G, Tendolkar I (2001) Integrated brain activity in medial temporal and prefrontal areas predicts subsequent memory performance: human declarative memory formation at the system level. Brain Res Bull 55:1–9
Ferreira MA, O’Donovan MC, Meng YA, Jones IR, Ruderfer DM, Jones L, Fan J, Kirov G, Perlis RH, Green EK, Smoller JW, Grozeva D, Stone J, Nikolov I, Chambert K, Hamshere ML, Nimgaonkar VL, Moskvina V, Thase ME, Caesar S, Sachs GS, Franklin J, Gordon-Smith K, Ardlie KG, Gabriel SB, Fraser C, Blumenstiel B, Defelice M, Breen G, Gill M, Morris DW, Elkin A, Muir WJ, McGhee KA, Williamson R, MacIntyre DJ, MacLean AW, St CD, Robinson M, Van Beck M, Pereira AC, Kandaswamy R, McQuillin A, Collier DA, Bass NJ, Young AH, Lawrence J, Ferrier IN, Anjorin A, Farmer A, Curtis D, Scolnick EM, McGuffin P, Daly MJ, Corvin AP, Holmans PA, Blackwood DH, Gurling HM, Owen MJ, Purcell SM, Sklar P, Craddock N (2008) Collaborative genome-wide association analysis supports a role for ANK3 and CACNA1C in bipolar disorder. Nat Genet 40:1056–1058
Goldberg JF, Chengappa KN (2009) Identifying and treating cognitive impairment in bipolar disorder. Bipolar Disord 11(Suppl 2):123–137
Goring HH, Terwilliger JD, Blangero J (2001) Large upward bias in estimation of locus-specific effects from genomewide scans. Am J Hum Genet 69:1357–1369
Green EK, Grozeva D, Jones I, Jones L, Kirov G, Caesar S, Gordon-Smith K, Fraser C, Forty L, Russell E, Hamshere ML, Moskvina V, Nikolov I, Farmer A, McGuffin P, Holmans PA, Owen MJ, O’Donovan MC, Craddock N (2010) The bipolar disorder risk allele at CACNA1C also confers risk of recurrent major depression and of schizophrenia. Mol Psychiatry 15:1016–1022
Hammar A, Ardal G (2009) Cognitive functioning in major depression–a summary. Front Hum Neurosci 3:26
Hill SK, Harris MS, Herbener ES, Pavuluri M, Sweeney JA (2008) Neurocognitive allied phenotypes for schizophrenia and bipolar disorder. Schizophr Bull 34:743–759
Jiang M, Swann JW (2005) A role for L-type calcium channels in the maturation of parvalbumin-containing hippocampal interneurons. Neuroscience 135:839–850
Jogia J, Ruberto G, Lelli-Chiesa G, Vassos E, Maieru M, Tatarelli R, Girardi P, Collier D, Frangou S (2011) The impact of the CACNA1C gene polymorphism on frontolimbic function in bipolar disorder. Mol Psychiatry 16:1070–1071
Kircher T, Krug A, Markov V, Whitney C, Krach S, Zerres K, Eggermann T, Stocker T, Shah NJ, Treutlein J, Nothen MM, Becker T, Rietschel M (2009) Genetic variation in the schizophrenia-risk gene neuregulin 1 correlates with brain activation and impaired speech production in a verbal fluency task in healthy individuals. Hum Brain Mapp 30:3406–3416
Kircher TT, Erb M, Grodd W, Leube DT (2005) Cortical activation during cholinesterase-inhibitor treatment in Alzheimer disease: preliminary findings from a pharmaco-fMRI study. Am J Geriatr Psychiatry 13:1006–1013
Konradi C, Zimmerman EI, Yang CK, Lohmann KM, Gresch P, Pantazopoulos H, Berretta S, Heckers S (2011) Hippocampal interneurons in bipolar disorder. Arch Gen Psychiatry 68:340–350
Krabbendam L, Arts B, van Os J, Aleman A (2005) Cognitive functioning in patients with schizophrenia and bipolar disorder: a quantitative review. Schizophr Res 80:137–149
Krach S, Jansen A, Krug A, Markov V, Thimm M, Sheldrick AJ, Eggermann T, Zerres K, Stocker T, Shah NJ, Kircher T (2010) COMT genotype and its role on hippocampal-prefrontal regions in declarative memory. Neuroimage 53:978–984
Krug A, Krach S, Jansen A, Nieratschker V, Witt SH, Shah NJ, Nothen MM, Rietschel M, Kircher T (2013) The effect of neurogranin on neural correlates of episodic memory encoding and retrieval. Schizophr Bull 39:141–150
Krug A, Markov V, Krach S, Jansen A, Zerres K, Eggermann T, Stocker T, Shah NJ, Nothen MM, Treutlein J, Rietschel M, Kircher T (2010) The effect of Neuregulin 1 on neural correlates of episodic memory encoding and retrieval. Neuroimage 53:985–991
Krug A, Markov V, Leube D, Zerres K, Eggermann T, Nothen MM, Skowronek MH, Rietschel M, Kircher T (2008) Genetic variation in the schizophrenia-risk gene neuregulin 1 correlates with personality traits in healthy individuals. Eur Psychiatry 23:344–349
Krug A, Markov V, Sheldrick A, Krach S, Jansen A, Zerres K, Eggermann T, Stocker T, Shah NJ, Kircher T (2009) The effect of the COMT val(158)met polymorphism on neural correlates of semantic verbal fluency. Eur Arch Psychiatry Clin Neurosci 259:459–465
Krug A, Nieratschker V, Markov V, Krach S, Jansen A, Zerres K, Eggermann T, Stocker T, Shah NJ, Treutlein J, Muhleisen TW, Kircher T (2010) Effect of CACNA1C rs1006737 on neural correlates of verbal fluency in healthy individuals. Neuroimage 49:1831–1836
Liu Y, Blackwood DH, Caesar S, de Geus EJ, Farmer A, Ferreira MA, Ferrier IN, Fraser C, Gordon-Smith K, Green EK, Grozeva D, Gurling HM, Hamshere ML, Heutink P, Holmans PA, Hoogendijk WJ, Hottenga JJ, Jones L, Jones IR, Kirov G, Lin D, McGuffin P, Moskvina V, Nolen WA, Perlis RH, Posthuma D, Scolnick EM, Smit AB, Smit JH, Smoller JW, St Clair D, van Dyck R, Verhage M, Willemsen G, Young AH, Zandbelt T, Boomsma DI, Craddock N, O’Donovan MC, Owen MJ, Penninx BW, Purcell S, Sklar P, Sullivan PF (2011) Meta-analysis of genome-wide association data of bipolar disorder and major depressive disorder. Mol Psychiatry 16:2–4
Lehrl S, Triebig G, Fischer B (1995) Multiple choice vocabulary test MWT as a valid and short test to estimate premorbid intelligence. Acta Neurol Scand 91:335–345
Marazziti D, Consoli G, Picchetti M, Carlini M, Faravelli L (2010) Cognitive impairment in major depression. Eur J Pharmacol 626:83–86
Markov V, Krug A, Krach S, Jansen A, Eggermann T, Zerres K, Stocker T, Shah NJ, Nothen MM, Treutlein J, Rietschel M, Kircher T (2010) Impact of schizophrenia-risk gene dysbindin 1 on brain activation in bilateral middle frontal gyrus during a working memory task in healthy individuals. Hum Brain Mapp 31:266–275
Modirrousta M, Fellows LK (2008) Medial prefrontal cortex plays a critical and selective role in ‘feeling of knowing’ meta-memory judgments. Neuropsychologia 46:2958–2965
Moosmang S, Haider N, Klugbauer N, Adelsberger H, Langwieser N, Muller J, Stiess M, Marais E, Schulla V, Lacinova L, Goebbels S, Nave KA, Storm DR, Hofmann F, Kleppisch T (2005) Role of hippocampal Cav1.2 Ca2+ channels in NMDA receptor-independent synaptic plasticity and spatial memory. J Neurosci 25:9883–9892
Moosmang S, Lenhardt P, Haider N, Hofmann F, Wegener JW (2005) Mouse models to study L-type calcium channel function. Pharmacol Ther 106:347–355
Moskvina V, Craddock N, Holmans P, Nikolov I, Pahwa JS, Green E, Owen MJ, O’Donovan MC (2009) Gene-wide analyses of genome-wide association data sets: evidence for multiple common risk alleles for schizophrenia and bipolar disorder and for overlap in genetic risk. Mol Psychiatry 14:252–260
Nyegaard M, Demontis D, Foldager L, Hedemand A, Flint TJ, Sorensen KM, Andersen PS, Nordentoft M, Werge T, Pedersen CB, Hougaard DM, Mortensen PB, Mors O, Borglum AD (2010) CACNA1C (rs1006737) is associated with schizophrenia. Mol Psychiatry 15:119–121
Oldfield RC (1971) The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9:97–113
Osuji IJ, Cullum CM (2005) Cognition in bipolar disorder. Psychiatr Clin North Am 28:427–441
Paulus FM, Bedenbender J, Krach S, Pyka M, Krug A, Sommer J, Mette M, Nöthen MM, Witt SH, Rietschel M, Kircher T, Jansen A (2013) Association of rs1006737 in CACNA1C with alterations in prefrontal activation and fronto-hippocampal connectivity. Hum Brain Mapp. doi:10.1002/hbm.22244 [Epub ahead of print]
Quraishi S, Frangou S (2002) Neuropsychology of bipolar disorder: a review. J Affect Disord 72:209–226
Quraishi S, Walshe M, McDonald C, Schulze K, Kravariti E, Bramon E, Morris RG, Murray RM, Toulopoulou T (2009) Memory functioning in familial bipolar I disorder patients and their relatives. Bipolar Disord 11:209–214
Ragland JD, Gur RC, Valdez J, Turetsky BI, Elliott M, Kohler C, Siegel S, Kanes S, Gur RE (2004) Event-related fMRI of frontotemporal activity during word encoding and recognition in schizophrenia. Am J Psychiatry 161:1004–1015
Ragland JD, Laird AR, Ranganath C, Blumenfeld RS, Gonzales SM, Glahn DC (2009) Prefrontal activation deficits during episodic memory in schizophrenia. Am J Psychiatry 166:863
Ranganath C, Minzenberg MJ, Ragland JD (2008) The cognitive neuroscience of memory function and dysfunction in schizophrenia. Biol Psychiatry 64:18–25
Ripke S, Sanders AR, Kendler KS, Levinson DF, Sklar P, Holmans PA, Lin DY, Duan J, Ophoff RA, Andreassen OA, Scolnick E, Cichon S, St Clair D, Corvin A, Gurling H, Werge T, Rujescu D, Blackwood DH, Pato CN, Malhotra AK, Purcell S, Dudbridge F, Neale BM, Rossin L, Visscher PM, Posthuma D, Ruderfer DM, Fanous A, Stefansson H, Steinberg S, Mowry BJ, Golimbet V, De Hert M, Jonsson EG, Bitter I, Pietilainen OP, Collier DA, Tosato S, Agartz I, Albus M, Alexander M, Amdur RL, Amin F, Bass N, Bergen SE, Black DW, Borglum AD, Brown MA, Bruggeman R, Buccola NG, Byerley WF, Cahn W, Cantor RM, Carr VJ, Catts SV, Choudhury K, Cloninger CR, Cormican P, Craddock N, Danoy PA, Datta S, de Haan L, Demontis D, Dikeos D, Djurovic S, Donnelly P, Donohoe G, Duong L, Dwyer S, Fink-Jensen A, Freedman R, Freimer NB, Friedl M, Georgieva L, Giegling I, Gill M, Glenthoj B, Godard S, Hamshere M, Hansen M, Hansen T, Hartmann AM, Henskens FA, Hougaard DM, Hultman CM, Ingason A, Jablensky AV, Jakobsen KD, Jay M, Jurgens G, Kahn RS, Keller MC, Kenis G, Kenny E, Kim Y, Kirov GK, Konnerth H, Konte B, Krabbendam L, Krasucki R et al (2011) Genome-wide association study identifies five new schizophrenia loci. Nat Genet 43:969–976
Robinson-Long M, Eslinger PJ, Wang J, Meadowcroft M, Yang QX (2009) Functional MRI evidence for distinctive binding and consolidation pathways for face-name associations: analysis of activation maps and BOLD response amplitudes. Top Magn Reson Imaging 20:271–278
Rossignol E (2011) Genetics and function of neocortical GABAergic interneurons in neurodevelopmental disorders. Neural Plast 2011:649325
Schnyer DM, Nicholls L, Verfaellie M (2005) The role of VMPC in metamemorial judgments of content retrievability. J Cogn Neurosci 17:832–846
Sklar P, Smoller JW, Fan J, Ferreira MA, Perlis RH, Chambert K, Nimgaonkar VL, McQueen MB, Faraone SV, Kirby A, de Bakker PI, Ogdie MN, Thase ME, Sachs GS, Todd-Brown K, Gabriel SB, Sougnez C, Gates C, Blumenstiel B, Defelice M, Ardlie KG, Franklin J, Muir WJ, McGhee KA, MacIntyre DJ, McLean A, VanBeck M, McQuillin A, Bass NJ, Robinson M, Lawrence J, Anjorin A, Curtis D, Scolnick EM, Daly MJ, Blackwood DH, Gurling HM, Purcell SM (2008) Whole-genome association study of bipolar disorder. Mol Psychiatry 13:558–569
Summerfield C, Mangels JA (2005) Functional coupling between frontal and parietal lobes during recognition memory. NeuroReport 16:117–122
Thimm M, Kircher T, Kellermann T, Markov V, Krach S, Jansen A, Zerres K, Eggermann T, Stocker T, Shah NJ, Nothen MM, Rietschel M, Witt SH, Mathiak K, Krug A (2011) Effects of a CACNA1C genotype on attention networks in healthy individuals. Psychol Med 41:1551–1561
Thimm M, Krug A, Markov V, Krach S, Jansen A, Zerres K, Eggermann T, Stocker T, Shah NJ, Nothen MM, Rietschel M, Kircher T (2010) The impact of dystrobrevin-binding protein 1 (DTNBP1) on neural correlates of episodic memory encoding and retrieval. Hum Brain Mapp 31:203–209
Werner NS, Meindl T, Materne J, Engel RR, Huber D, Riedel M, Reiser M, Hennig-Fast K (2009) Functional MRI study of memory-related brain regions in patients with depressive disorder. J Affect Disord 119:124–131
Wessa M, Linke J, Witt SH, Nieratschker V, Esslinger C, Kirsch P, Grimm O, Hennerici MG, Gass A, King AV, Rietschel M (2010) The CACNA1C risk variant for bipolar disorder influences limbic activity. Mol Psychiatry 15:1126–1127
White JA, McKinney BC, John MC, Powers PA, Kamp TJ, Murphy GG (2008) Conditional forebrain deletion of the L-type calcium channel Ca V 1.2 disrupts remote spatial memories in mice. Learn Mem 15:1–5
Woodside BL, Borroni AM, Hammonds MD, Teyler TJ (2004) NMDA receptors and voltage-dependent calcium channels mediate different aspects of acquisition and retention of a spatial memory task. Neurobiol Learn Mem 81:105–114
Acknowledgments
This work was supported by grants from the Federal Ministry of Education and Research (BMBF Grant No. 01GO0204 and NGFN-Plus Grant No. 01GS08147) and the German Research Foundation (DFG, Grant No. KR 3822/2-1). The BMBF and DFG had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Conflict of interest
All authors report no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Axel Krug and Stephanie H. Witt share first authorship.
Rights and permissions
About this article
Cite this article
Krug, A., Witt, S.H., Backes, H. et al. A genome-wide supported variant in CACNA1C influences hippocampal activation during episodic memory encoding and retrieval. Eur Arch Psychiatry Clin Neurosci 264, 103–110 (2014). https://doi.org/10.1007/s00406-013-0428-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00406-013-0428-x