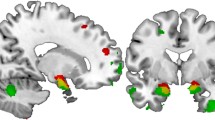
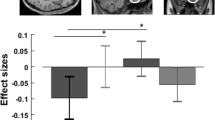
Abstract
Affective deficits are one common denominator of schizophrenia (SZ), bipolar disorder (BD) and obsessive compulsive disorder (OCD) with the amygdala indicated as one of the major structures involved in emotion regulation. Previous findings of differences in amygdala volume between healthy controls and patients with SZ, BD or OCD diverge with respect to the affected hemisphere, size and direction of the effect. Variability in the CACNA1C gene has been linked to BD, SZ as well as structural and functional variation in the amygdala in healthy people and patients with BD. We were interested to investigate whether amygdala volumes differ between hemispheres, diagnostic or genotype groups, and whether any interactive effects exist. We combined genotyping of SNP rs1006737 in CACNA1C with structural MRI measurements of relative gray matter (GM) amygdala volume in patients with SZ, BD or OCD as well as healthy controls (N Total = 72). The CACNA1C genotype showed a significant effect on relative GM amygdala volume in patients with SZ. There was a significant left versus right relative GM amygdala volume decrease in patients with SZ or BD. The effects of hemisphere and diagnosis (controls vs. patients with SZ) on relative GM amygdala volume were genotype specific. Our data suggest that the CACNA1C genotype may account for some heterogeneity in the effects of hemisphere and diagnosis on amygdala volume when comparing patients with SZ and controls and point to disturbed Ca2+-signaling as a plausible mechanism contributing to the pathology in patients with SZ.



Similar content being viewed by others
References
Arnone D, Cavanagh J, Gerber D, Lawrie SM, Ebmeier KP, McIntosh AM (2009) Magnetic resonance imaging studies in bipolar disorder and schizophrenia: meta-analysis. Br J Psychiatr 195:194–201
Blond BN, Fredericks CA, Blumberg HP (2012) Functional neuroanatomy of bipolar disorder: structure, function, and connectivity in an amygdala-anterior paralimbic neural system. Bipolar Disord 14:340–355
Honea R, Crow TJ, Passingham D, Mackay CE (2005) Regional deficits in brain volume in schizophrenia: a meta-analysis of voxel-based morphometry studies. Am J Psychiatr 162:2233–2245
Kwon JS, Shin YW, Kim CW, Kim YI, Youn T, Han MH et al (2003) Similarity and disparity of obsessive-compulsive disorder and schizophrenia in MR volumetric abnormalities of the hippocampus-amygdala complex. J Neurol Neurosurg Psychiatr 74:962–964
Menzies L, Chamberlain SR, Laird AR, Thelen SM, Sahakian BJ, Bullmore ET (2008) Integrating evidence from neuroimaging and neuropsychological studies of obsessive-compulsive disorder: the orbitofronto-striatal model revisited. Neurosci Biobehav Rev 32:525–549
Milad MR, Rauch SL (2012) Obsessive-compulsive disorder: beyond segregated cortico-striatal pathways. Trends Cogn Sci 16:43–51
Rotge JY, Guehl D, Dilharreguy B, Tignol J, Bioulac B, Allard M et al (2009) Meta-analysis of brain volume changes in obsessive-compulsive disorder. Biol Psychiatr 65:75–83
Strakowski SM, Delbello MP, Adler CM (2005) The functional neuroanatomy of bipolar disorder: a review of neuroimaging findings. Mol Psychiatr 10:105–116
Strakowski SM, Adler CM, Almeida J, Altshuler LL, Blumberg HP, Chang KD et al (2012) The functional neuroanatomy of bipolar disorder: a consensus model. Bipolar Disord 14:313–325
Szeszko PR, Robinson D, Alvir JM, Bilder RM, Lencz T, Ashtari M et al (1999) Orbital frontal and amygdala volume reductions in obsessive-compulsive disorder. Arch Gen Psychiatr 56:913–919
Townsend J, Altshuler LL (2012) Emotion processing and regulation in bipolar disorder: a review. Bipolar Disord 14:326–339
Yoshida T, McCarley RW, Nakamura M, Lee K, Koo MS, Bouix S et al (2009) A prospective longitudinal volumetric MRI study of superior temporal gyrus gray matter and amygdala-hippocampal complex in chronic schizophrenia. Schizophr Res 113:84–94
Jogia J, Ruberto G, Lelli-Chiesa G, Vassos E, Maieru M, Tatarelli R et al (2011) The impact of the CACNA1C gene polymorphism on frontolimbic function in bipolar disorder. Mol Psychiatr 16:1070–1071
Perrier E, Pompei F, Ruberto G, Vassos E, Collier D, Frangou S (2011) Initial evidence for the role of CACNA1C on subcortical brain morphology in patients with bipolar disorder. Eur Psychiatr 26:135–137
Wessa M, Linke J, Witt SH, Nieratschker V, Esslinger C, Kirsch P et al (2010) The CACNA1C risk variant for bipolar disorder influences limbic activity. Mol Psychiatr 15:1126–1127
Tesli M, Skatun KC, Ousdal OT, Brown AA, Thoresen C, Agartz I et al (2013) CACNA1C risk variant and amygdala activity in bipolar disorder, schizophrenia and healthy controls. PLoS ONE 8:e56970
Green EK, Grozeva D, Jones I, Jones L, Kirov G, Caesar S et al (2010) The bipolar disorder risk allele at CACNA1C also confers risk of recurrent major depression and of schizophrenia. Mol Psychiatr 15:1016–1022
Nyegaard M, Demontis D, Foldager L, Hedemand A, Flint TJ, Sorensen KM et al (2010) CACNA1C (rs1006737) is associated with schizophrenia. Mol Psychiatr 15:119–121
Ferreira MA, O’Donovan MC, Meng YA, Jones IR, Ruderfer DM, Jones L et al (2008) Collaborative genome-wide association analysis supports a role for ANK3 and CACNA1C in bipolar disorder. Nat Genet 40:1056–1058
Sklar P, Smoller JW, Fan J, Ferreira MA, Perlis RH, Chambert K et al (2008) Whole-genome association study of bipolar disorder. Mol Psychiatr 13:558–569
Erk S, Meyer-Lindenberg A, Schnell K, Opitz von Boberfeld C, Esslinger C, Kirsch P et al (2010) Brain function in carriers of a genome-wide supported bipolar disorder variant. Arch Gen Psychiatr 67:803–811
Roussos P, Giakoumaki SG, Georgakopoulos A, Robakis NK, Bitsios P (2011) The CACNA1C and ANK3 risk alleles impact on affective personality traits and startle reactivity but not on cognition or gating in healthy males. Bipolar Disord 13:250–259
Strohmaier J, Amelang M, Hothorn LA, Witt SH, Nieratschker V, Gerhard D et al (2013) The psychiatric vulnerability gene CACNA1C and its sex-specific relationship with personality traits, resilience factors and depressive symptoms in the general population. Mol Psychiatr 18:607–613
Hafeman DM, Chang KD, Garrett AS, Sanders EM, Phillips ML (2012) Effects of medication on neuroimaging findings in bipolar disorder: an updated review. Bipolar Disord 14:375–410
Amunts K, Kedo O, Kindler M, Pieperhoff P, Mohlberg H, Shah NJ et al (2005) Cytoarchitectonic mapping of the human amygdala, hippocampal region and entorhinal cortex: intersubject variability and probability maps. Anat Embryol (Berl) 210:343–352
Brierley B, Shaw P, David AS (2002) The human amygdala: a systematic review and meta-analysis of volumetric magnetic resonance imaging. Brain Res Brain Res Rev 39:84–105
Pruessner JC, Li LM, Serles W, Pruessner M, Collins DL, Kabani N et al (2000) Volumetry of hippocampus and amygdala with high-resolution MRI and three-dimensional analysis software: minimizing the discrepancies between laboratories. Cereb Cortex 10:433–442
Bigos KL, Mattay VS, Callicott JH, Straub RE, Vakkalanka R, Kolachana B et al (2010) Genetic variation in CACNA1C affects brain circuitries related to mental illness. Arch Gen Psychiatr 67:939–945
Zhang Q, Shen Q, Xu Z, Chen M, Cheng L, Zhai J et al (2012) The effects of CACNA1C gene polymorphism on spatial working memory in both healthy controls and patients with schizophrenia or bipolar disorder. Neuropsychopharmacology 37:677–684
Hamshere ML, Walters JT, Smith R, Richards AL, Green E, Grozeva D, et al. (2013) Genome-wide significant associations in schizophrenia to ITIH3/4, CACNA1C and SDCCAG8, and extensive replication of associations reported by the Schizophrenia PGC. Mol Psychiatr 18:708–712
Bora E, Fornito A, Radua J, Walterfang M, Seal M, Wood SJ et al (2011) Neuroanatomical abnormalities in schizophrenia: a multimodal voxelwise meta-analysis and meta-regression analysis. Schizophr Res 127:46–57
Ellison-Wright I, Glahn DC, Laird AR, Thelen SM, Bullmore E (2008) The anatomy of first-episode and chronic schizophrenia: an anatomical likelihood estimation meta-analysis. Am J Psychiatr 165:1015–1023
Kong L, Bachmann S, Thomann PA, Essig M, Schroder J (2012) Neurological soft signs and gray matter changes: a longitudinal analysis in first-episode schizophrenia. Schizophr Res 134:27–32
Meisenzahl EM, Koutsouleris N, Bottlender R, Scheuerecker J, Jager M, Teipel SJ et al (2008) Structural brain alterations at different stages of schizophrenia: a voxel-based morphometric study. Schizophr Res 104:44–60
Vita A, De Peri L, Silenzi C, Dieci M (2006) Brain morphology in first-episode schizophrenia: a meta-analysis of quantitative magnetic resonance imaging studies. Schizophr Res 82:75–88
Watson DR, Anderson JM, Bai F, Barrett SL, McGinnity TM, Mulholland CC et al (2012) A voxel based morphometry study investigating brain structural changes in first episode psychosis. Behav Brain Res 227:91–99
Witthaus H, Kaufmann C, Bohner G, Ozgurdal S, Gudlowski Y, Gallinat J et al (2009) Gray matter abnormalities in subjects at ultra-high risk for schizophrenia and first-episode schizophrenic patients compared to healthy controls. Psychiatr Res 173:163–169
Hulshoff Pol HE, Schnack HG, Posthuma D, Mandl RC, Baare WF, van Oel C et al (2006) Genetic contributions to human brain morphology and intelligence. J Neurosci 26:10235–10242
Peper JS, Schnack HG, Brouwer RM, Van Baal GC, Pjetri E, Szekely E et al (2009) Heritability of regional and global brain structure at the onset of puberty: a magnetic resonance imaging study in 9-year-old twin pairs. Hum Brain Mapp 30:2184–2196
Kobrinsky E, Tiwari S, Maltsev VA, Harry JB, Lakatta E, Abernethy DR et al (2005) Differential role of the alpha1C subunit tails in regulation of the Cav1.2 channel by membrane potential, beta subunits, and Ca2+ ions. J Biol Chem 280:12474–12485
Dolmetsch RE, Pajvani U, Fife K, Spotts JM, Greenberg ME (2001) Signaling to the nucleus by an L-type calcium channel-calmodulin complex through the MAP kinase pathway. Science 294:333–339
Monfils MH, Cowansage KK, LeDoux JE (2007) Brain-derived neurotrophic factor: linking fear learning to memory consolidation. Mol Pharmacol 72:235–237
Wolf C, Linden DE (2012) Biological pathways to adaptability–interactions between genome, epigenome, nervous system and environment for adaptive behavior. Genes Brain Behav 11:3–28
Langwieser N, Christel CJ, Kleppisch T, Hofmann F, Wotjak CT, Moosmang S (2010) Homeostatic switch in hebbian plasticity and fear learning after sustained loss of Cav1.2 calcium channels. J Neurosci 30:8367–8375
Shinnick-Gallagher P, McKernan MG, Xie J, Zinebi F (2003) L-type voltage-gated calcium channels are involved in the in vivo and in vitro expression of fear conditioning. Ann NY Acad Sci 985:135–149
Dao DT, Mahon PB, Cai X, Kovacsics CE, Blackwell RA, Arad M et al (2010) Mood disorder susceptibility gene CACNA1C modifies mood-related behaviors in mice and interacts with sex to influence behavior in mice and diagnosis in humans. Biol Psychiatr 68:801–810
Mogilnicka E, Czyrak A, Maj J (1987) Dihydropyridine calcium channel antagonists reduce immobility in the mouse behavioral despair test; antidepressants facilitate nifedipine action. Eur J Pharmacol 138:413–416
Sinnegger-Brauns MJ, Hetzenauer A, Huber IG, Renstrom E, Wietzorrek G, Berjukov S et al (2004) Isoform-specific regulation of mood behavior and pancreatic beta cell and cardiovascular function by L-type Ca2+ channels. J Clin Invest 113:1430–1439
Franke B, Vasquez AA, Veltman JA, Brunner HG, Rijpkema M, Fernandez G (2010) Genetic variation in CACNA1C, a gene associated with bipolar disorder, influences brainstem rather than gray matter volume in healthy individuals. Biol Psychiatr 68:586–588
Soeiro-de-Souza MG, Otaduy MC, Dias CZ, Bio DS, Machado-Vieira R, Moreno RA (2012) The impact of the CACNA1C risk allele on limbic structures and facial emotions recognition in bipolar disorder subjects and healthy controls. J Affect Disord 141:94–101
Wang F, McIntosh AM, He Y, Gelernter J, Blumberg HP (2011) The association of genetic variation in CACNA1C with structure and function of a frontotemporal system. Bipolar Disord 13:696–700
Giegling I, Genius J, Benninghoff J, Rujescu D (2010) Genetic findings in schizophrenia patients related to alterations in the intracellular Ca-homeostasis. Prog Neuropsychopharmacol Biol Psychiatr 34:1375–1380
Glessner JT, Reilly MP, Kim CE, Takahashi N, Albano A, Hou C et al (2010) Strong synaptic transmission impact by copy number variations in schizophrenia. Proc Natl Acad Sci USA 107:10584–10589
Lidow MS (2003) Calcium signaling dysfunction in schizophrenia: a unifying approach. Brain Res Brain Res Rev 43:70–84
Rushlow WJ, Seah C, Sutton LP, Bjelica A, Rajakumar N (2009) Antipsychotics affect multiple calcium calmodulin dependent proteins. Neuroscience 161:877–886
Stefansson H, Ophoff RA, Steinberg S, Andreassen OA, Cichon S, Rujescu D et al (2009) Common variants conferring risk of schizophrenia. Nature 460:744–747
Acknowledgments
We thank Patrick Menzel for assistance with the MRI data processing. This work was supported by a grant from the Competence Network Schizophrenia and partially supported by the Deutsche Forschungsgemeinschaft (DFG) via the Clinical Research Group 241 “Genotype-phenotype relationships and neurobiology of the longitudinal course of psychosis” (http://www.kfo241.de; Grant Number GR 1950/5-1) to OG.
Conflict of interest
CW, HM, TSA, AR, HS, AS and PF declare no biomedical financial interest or potential conflicts of interest. TW is a member of a speaker bureau for Alpine Biomed, AstraZeneca, Bristol-Meyers-Squibb, Eli Lilly, Jansen Cilag and Sanofi-Synthelabo and has received a research grant from AstraZeneca, I3G and AOK (health insurance company). OG was honorary speaker for the following companies: AstraZeneca, Bristol-Meyers-Squibb, Jansen Cilag, Lilly and Otsuka, has been invited to scientific congresses by AstraZeneca, Jansen Cilag, Pfizer and has received a research grant from Servier.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wolf, C., Mohr, H., Schneider-Axmann, T. et al. CACNA1C genotype explains interindividual differences in amygdala volume among patients with schizophrenia. Eur Arch Psychiatry Clin Neurosci 264, 93–102 (2014). https://doi.org/10.1007/s00406-013-0427-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00406-013-0427-y