Abstract
Background
The alignment between objective scores and patient-reported outcome measures (PROMs) is underexplored. This study aimed to assess changes in Nasal Polyp Score (NPS) and Sino-Nasal Outcome Test (SNOT) scores in chronic rhinosinusitis with nasal polyps (CRSwNP) patients undergoing dupilumab treatment and explore correlations between these scores.
Methods
CRSwNP patients received dupilumab therapy for six months. SNOT-20 German Adapted Version (GAV)/SNOT-22 scores were assessed weekly, and NPS was measured at baseline and after one, three, and six months. Correlations were analyzed using Spearman’s rank correlation and regression analysis.
Results
69 patients were included. After one, three and six months of dupilumab therapy, SNOT and NPS scores improved significantly. Correlation analysis of SNOT and NPS showed significant correlations only within the nasal subscores, along with a weak trend for SNOT-20. Absolute changes over time lacked significance. However, correlation analysis revealed significant associations between relative changes in SNOT score and NPS, irrespective of timing, and when stratified by baseline NPS of 8, 6, and 4 (r = -0.54, p = 0.01; r = -0.44, p < 0.001; r = -0.7, p < 0.001). This was supported by linear regression modeling, suggesting potential predictive capability of NPS reduction on relative SNOT score improvement.
Conclusion
Dupilumab therapy significantly improved subjective and objective CRSwNP scores, exhibiting weak correlations in absolute values for nasal subscores. Furthermore, evidence indicated a correlation between relative changes in SNOT score and NPS, substantiated by predictive capability. This might be due to subjective perception variability, highlighting the suitability of relative change correlation analysis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Chronic rhinosinusitis with nasal polyps (CRSwNP) is a prevalent and heterogeneous inflammatory disease, often associated with a significant subjective burden for patients [1, 2]. Patients experience nasal congestion, olfactory loss and rhinorrhea resulting in a reduced quality of life [3, 4].
Advances in understanding the underlying pathophysiology of CRSwNP have led to the increasing importance of immunomodulatory therapies with biologics for CRSwNP patients [5,6,7,8]. Notably, the approval of dupilumab (anti-interleukin-4 receptor alpha monoclonal antibody) by the US Food and Drug Administration and the European Medicines Agency in 2019 has revolutionized treatment options for CRSwNP patients [9]. Studies have shown that therapy not only improves nasal-specific symptoms but also other health-related aspects such as decreased nocturnal awakenings, increased concentration, and less frustration [10, 11].
Various assessment tools are accessible for quantifying therapy responses.
The endoscopically determined nasal polyp score (NPS), often referred to as the total polyp score (TPS), is widely used in clinical trials [12,13,14]. The addition of a PROM to objective endoscopic evaluation methods is strongly recommended to assess treatment response comprehensively [15]. Patient-Related Outcome Measures (PROMs) have the advantage of capturing individual distress and subjective symptomatology, which are crucial criteria for health insurance approval, given the high cost of dupilumab therapy [16, 17]. One of the most qualitatively representative tools that can be utilized is the Sino-Nasal Outcome Test (SNOT), a well-established questionnaire [18].
The extent to which different nasal polyp scoring systems align with PROMs has not been thoroughly explored in existing literature. The European position paper on rhinosinusitis and nasal polyps 2020 emphasizes the lack of literature investigating the correlation between patient-reported and objective outcome measures for CRS [9]. As nasal polyp grading gaining importance as a primary outcome in pharmacological studies and for health insurance approval, several researchers have examined the relationship between objective measures and patient-reported outcome measures [15, 19]. With the predominant conclusion being that the predictive ability of current scoring systems for PROMs is poor and not significant. This underscores the need for further comprehensive research on this topic.
The aim of this study was to evaluate the changes in NPS and SNOT scores in patients with CRSwNP undergoing dupilumab therapy and to investigate the potential correlation between NPS and SNOT scores.
Methods
Data from two cohorts, the retrospective monocentric cohort study DUPIPOLYP (n = 43) and the prospective observational cohort study IMMUNOPOLYP (n = 26), collected between June 2020 and November 2022, were analyzed. All participants had signed the written general informed consent form of the University Hospital Zurich (Switzerland) or the study-specific consent form for the IMMUNOPOLYP study. These clinical studies were both conducted with approval from the cantonal ethics committee (BASEC-No. 2021 − 01213 and 2020–02955).
Included in this study were adult patients (≥ 18 years), diagnosed with refractory CRSwNP according to EPOS 2020 [9]. These patients either had a history of previous surgery or were not eligible for surgery. Data from patients who prematurely terminated the study were also included in the analyses.
All patients received Dupixent® (Sanofi) injections subcutaneously every two weeks (300 mg/2 ml dupilumab), alongside nasal saline rinsing and topical steroids. Baseline NPS severity was assessed at first injection, with further consultations at one, three, and six months. For DUPIPOLYP patients additional examinations were conducted one week post-study commencement.
Nasal polyp evaluation involved meticulous video-recorded assessments, with NPS graded based on extension (assessed by summing scores from the right and left nostrils, on a scale of 0 to 8; higher scores indicating a more severe condition) [20].
Patients were instructed to complete the SNOT on a smartphone or computer weekly (SNOT-20 German Adapted Version (GAV) n = 43, SNOT-22 n = 26), and the data were directly transferred to our clinic database (ENT Statistics, Innoforce, Switzerland). For the analyses, SNOT scores completed within ± 7 days of the consultation were evaluated. The SNOT-20 GAV is a validated instrument for assessing symptoms of colds and upper respiratory diseases, consisting of 20 questions divided into three categories: “primary nasal symptoms” (PNS: 5 questions), “secondary rhinogenic symptoms” (SRS: 6 questions), and “general quality of life” (ALQ: 9 questions) [21, 22].
Symptomatology is classified as mild at 0–10 points, moderate at 11–40 points, moderately severe at 41–69 points, and severe at 51 points or more [23]. As a newly validated German version of the SNOT-22 was available during the study, we followed the international standard with 22 questions. The SNOT-22 test comprises 22 questions divided into five categories: “nasal symptoms,” “sleep,” “ear pain,” “function,” and “emotions” [24]. Symptomatology is considered mild at 8–20 points, moderate at 21–50 points, and severe at 51 points or above [25].
Statistical analysis
Parametric variables were analyzed using Student’s paired t-test, while non-parametric data were assessed via the Wilcoxon rank-sum test. Linear associations were examined using Spearman’s rank correlation, further validated by linear regression. Significance was set at p < 0.05. All analyses were conducted using R-Studio software (version 4.2.2.) [26].
Results
Patient characteristics
A total of 69 patients from the DUPIPOLYP (n = 43) and IMMUNOPOLYP (n = 26) studies were included in the analyses. The patient distribution comprised 23 females (33%) and 46 males (67%). The mean age at the initiation of therapy was calculated as 47.85 years (standard deviation (SD) +/- 12.97), ranging from the youngest patient being 24 years to the oldest 77 years. Seven patients terminated the study prematurely. The reasons for study discontinuation were as follows: unclear reasons (n = 3), relocation abroad (n = 1), worsening of symptoms (n = 1), intensified tinnitus (n = 1), and stress (n = 1).
Among all patients, 43 completed the SNOT-20 questionnaire, while the SNOT-22 questionnaire was completed by 26 patients. A baseline SNOT score was available for 54 out of the total 69 patients, and baseline NPS data were collected for the entire patient cohort.
Effects of dupilumab therapy on SNOT scores and NPS
The mean baseline SNOT score at study initiation was 43.3 points, with a SD of 16.8 points. One month after starting dupilumab therapy, the SNOT-20 score exhibited a significant improvement of 19 points (46.57%) (p < 0.001, CI 14.5–25.6), while the SNOT-22 score exhibited a decrease of 24.1 points (49.8%) (p < 0.001, CI 13.6–37.1) after the first month. Over the course of 3 and 6 months, the SNOT-20 score demonstrated reductions of 19.9 and 22.7 points, respectively, while the SNOT-22 score displayed corresponding declines of 33.8 and 35.7 points. Importantly, in 30 out of 33 patients (90.9%), the SNOT score exhibited a reduction after 3 months relative to the baseline.
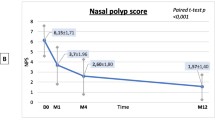
At baseline, the NPS displayed a mean value of 4.97 (median: 6, min: 1, max: 8). Following one month of therapy, a substantial reduction of 3 points (50%) was observed in the NPS (p < 0.001, CI 2-2.5). The NPS demonstrated further declines of 2.03 and 2.69 points over the subsequent 3 and 6 months, respectively. Notably, in 50 out of 54 patients (92.6%), an improvement in the NPS was evident after 3 months. In conclusion, a significant trend of improving objective and subjective scores was evident during dupilumab therapy. The results are displayed in Table 1; Fig. 1.
SNOT-20 GAV and SNOT-22 scores with the collected NPS under dupilumab therapy. SNOT-20 GAV or SNOT-22 score (blue) and NPS (green) at days 0, 28, 90, and 180 after initiation of therapy with dupilumab. The whole cohort together (left), DUPIPOLYP cohort (n = 43) (middle), IMMUNOPOLYP cohort (n = 26) (right). Significant differences in the means or medians of the boxplots from the day 0 reference group are indicated as follows: p < 0.05*, p < 0.01**, p < 0.001***. SNOT: Sino-Nasal Outcome Test, GAV: German Adapted Version, NPS: Nasal Polyp Score. (A) SNOT score at days 0, 28, 90, and 180 of the whole cohort (n = 69). (B) SNOT-20 GAV score at days 0, 28, 90, and 180 (n = 43). (C) SNOT-22 score at days 0, 28, 90, and 180 (n = 26). (D) NPS at days 0, 28, 90, and 180 of the whole cohort (n = 69). (E) NPS at days 0, 28, 90, and 180 of the DUPIPOLYP cohort (n = 43). (F) NPS at days 0, 28, 90, and 180 of the IMMUNOPOLYP cohort (n = 26)
Correlation of the absolute values of the SNOT score with the NPS
Correlating the absolute SNOT and NPS scores, regardless of the time point and therapy course, only the SNOT-20 score exhibited a linear, very weak trend (r = 0.17, p = 0.027). The SNOT-22 score did not show a significant correlation with the NPS, but fewer patient data were captured for this analysis (SNOT-20: 169 data points, SNOT-22: 77 data points) (Fig. 2a-b).
Correlation of the absolute values of the SNOT-20 GAV or SNOT-22 score to the NPS independent of the time of assessment. Spearman’s correlation of absolute values between SNOT-20 GAV (A) and SNOT-22 (B) and NPS regardless of the time of assessment. Spearman’s correlation of the absolute values of the SNOT-20 GAV PNS subscore (C), SNOT-22 nasal subscore (D), and the NPS at all time points. The linear trend is plotted as a red line with the 95% confidence interval as a green shadow. Spearman’s Rho as well as its significance are shown in the upper left of each. SNOT: Sino-Nasal Outcome Test, GAV: German Adapted Version, NPS: Nasal Polyp Score, PNS: Primary Nasal Symptoms. R = Spearman’s Rho, p = p-value
When analyzing the correlations between the SNOT subscores and NPS, significant correlations were observed exclusively for the PNS subscore in the SNOT-20 score (r = 0.28, p < 0.001) and the nasal subscore in the SNOT-22 score (r = 0.32, p = 0.005) (Fig. 2c and d).
Correlation of the absolute changes over time of the SNOT score with the NPS
Analyzing the absolute change over time did not yield significant results: Difference from Day 0 to Day 90 NPS vs. SNOT-20 (r = 0.14, p = 0.58), NPS vs. SNOT-22 (r = 0.065, p = 0.81), difference from Day 0 to Day 180 NPS vs. SNOT-20 (r = 0.0098, p = 0.97), NPS vs. SNOT-22 (r = 0.05, p = 0.86) (data not shown). In summary, the absolute change in NPS did not correlate with the absolute change in SNOT scores.
Correlation between the relative changes in SNOT score and NPS, irrespective of the timing
To assess the SNOT score independently of its baseline value, the relative differences between time points Day 0–27, 28–90, and 91–180 were computed and compared with the relative changes in the polyp score during these intervals, enabling a joint evaluation of the SNOT-20 and SNOT-22 scores. A significant positive correlation was identified (r = 0.29, p = 0.004). The analysis indicated that a complete reduction of nasal polyps by 100% corresponds to a statistically significant decrease of 32% in the SNOT score. A 50% decrease in polyps, was associated with a reduction of 25% in the SNOT score. Furthermore, even in cases where the NPS did not improve, there was an evident 18% improvement in the SNOT score. However, employing a linear regression model did not yield conclusive evidence for the influence of NPS reduction on SNOT score changes. The calculated regression coefficient, with a potential for predicting SNOT score improvement, is 0.141 (p = 0.12, SE = 0.09, y = 0.141*x+(-18.07)). In summary, they exhibit correlation but lack predictive capability. The findings are visually represented in Fig. 3.
Correlation of percentage values of SNOT-20/22 score to NPS independent of time point. Relative decrease/increase in SNOT score 20/22 (y-axis) correlated (Spearman) with the relative decrease in nasal polyp score (x-axis). Differences were calculated from days 0–28, 29–90, and 91–180, for each patient. Consequently, each patient contributed three data points to the analysis. The red line shows the linear trend with the 95% confidence interval as shading. Spearman’s Rho and its significance are shown in the top left corner of each case. SNOT: Sino-Nasal Outcome Test, NPS: Nasal Polyp Score. R = Spearman’s Rho, p = p-value
When analyzing the SNOT score using the same method described above but subdivided into its subscores, we observed that the PNS subscore displayed no correlation, as illustrated in Fig. 4a. Nonetheless, a marginal, but statistically significant improvement in the SRS and ALQ subscores was noted concurrently with a reduction in nasal polyps, as depicted in Fig. 4b and c. Conversely, when examining the SNOT-22 subscores, our investigation revealed an absence of correlation between the percentage difference in subscores and the relative reduction of the NPS (Fig. 4d-h).
Percentage decrease/increase in SNOT-20 GAV and SNOT-22 subscores correlated (Spearman) with percentage decrease in NPS. (A) SNOT-20 subscore “primary nasal symptoms”. (B) SNOT-20 subscore “secondary rhinogenic symptoms”. (C) SNOT-20 subscore “general quality of life”. (D) SNOT-22 subscore “nasal symptoms”. (E) SNOT-22 subscore “sleep”. (F) SNOT-22 subscore “earache”. (G) SNOT-22 subscore “function”. (H) SNOT-22 subscore “emotions”. Differences were calculated from day 0–28, 28–90, and 90–180. The red line shows the linear trend with the 95% confidence interval as shading. Spearman’s Rho as well as its significance are shown in the upper left corner of each case. SNOT: Sino-Nasal Outcome Test, GAV: German Adapted Version, NPS: Nasal Polyp Score. R = Spearman’s Rho, p = p-value
Correlation between the relative changes in SNOT and NPS, stratified by the baseline NPS
Since both the baseline SNOT score and the baseline NPS exhibit significant variability, patients were grouped based on the polyp grade at day 0, (NPS 8: 7 patients, NPS 6: 26 patients, NPS 4: 10 patients, NPS 2: 9 patients). Participants with NPS 7 (n = 4), 5 (n = 6), 3 (n = 4) und 1 (n = 3) were excluded from the analysis due to insufficient sample size.
The baseline SNOT score for each patient was set at 100% irrespective of its absolute value. Subsequently, the correlation between the changes in SNOT and NPS was examined. In all groups, a significant correlation between the relative change in SNOT score and the NPS was observed. The correlation was: r = -0.54 (p = 0.01), r = -0.44 (p < 0.001), and r = -0.7 (p < 0.001) for the groups with baseline NPS of 8, 6, and 4, respectively. However, no significant association between the two scores was found in the group with an NPS of 2 at the initiation of therapy (r = -0.17, p = 0.51). The results are displayed in Fig. 5a-d. The correlation was further validated through a linear regression model: NPS 8: y = 8.03x + 20.57 SE 2.75, p = 0.008, NPS 6: y = 5.5x + 52.42, SE 1.77, p = 0.003, NPS 4: y = 16.05x + 31.91, SE 3.8, p < 0.001. In summary, stratifying the scores by baseline NPS reveals correlations and even yields evidence of predictive capability.
Correlation of percentage of SNOT-20/22 score to NPS divided by baseline NPS at start of therapy. Patients were classified according to their NPS at baseline. The baseline SNOT score for each patient was set at 100%, irrespective of its absolute value. Then, the decrease/increase in SNOT-20/22 score was correlated to the percent decrease in nasal polyp score. The red line shows the linear trend with the 95% confidence interval as a shadow. SNOT: Sino-Nasal Outcome Test, NPS: Nasal Polyp Score. R = Spearman’s Rho, p = p-value. (A) All Patients with NPS 8 at baseline (n = 7). (B) All Patients with NPS 6 at baseline (n = 26). (C) All Patients with NPS 4 at baseline (n = 10). (D) All Patients with NPS 2 at baseline (n = 9)
Discussion
Main results
Patients responded rapidly to dupilumab therapy. Correlation analysis of absolute values revealed no significance. Specifically, a decrease in absolute NPS doesn’t necessarily correspond to a simultaneous reduction in the absolute SNOT-22 score. Similarly, the observed weak trend between NPS and SNOT-20 underscores its negligible relevance. Furthermore, analyzing absolute changes over time yielded no correlations. Our method for evaluating relative changes involves considering the percentage shift in scores in relation to the baseline. Relative changes in NPS and SNOT scores are statistically connected, yet regression analysis indicated non-significant predictive value. However, stratifying patients by baseline NPS revealed a significant correlation as well as predictive capability between NPS and relative SNOT score change in most groups (NPS 4, 6, 8).
Effect of dupilumab therapy on scores
Our findings align with existing evidence supporting the positive impact of dupilumab on SNOT and NPS scores [27, 28]. In our study, a significant average decrease of 24.1 points in the SNOT-22 score was observed after just one month. This reduction surpasses the minimal clinically important difference (MCID) reported by Hopkins et al. (-8.9 points) [29], and even exceeds the MCID of -12 points for medically managed CRS patients established by Phillips et al. [30]. Consequently, it can be concluded that Dupilumab not only demonstrated statistically significant enhancement but also yielded clinically meaningful improvement (as measured by PROMS).
Correlation analyses of absolute values
Even though objective scores and PROMs tend to decrease drastically under dupilumab therapy, the correlation between them has not been comprehensively explored in previous studies. Hence, Ta et al. 2021 conducted a systematic review examining the relationship between objective outcome measures and PROMs [15]. Nasal endoscopic ratings failed to exhibit any statistically significant correlations with PROMs. Consistent with these findings, other studies also arrived at a similar conclusion, indicating a lack of correlation between widely used endoscopic scoring systems and SNOT-22 scores [31, 32]. Aligning with these results, our analysis of absolute score values revealed no significant correlation between SNOT-22 score and NPS.
Recent research specifically focusing on dupilumab therapy in CRSwNP patients has demonstrated that NPS scoring correlates with objective measures, such as the SST-12. However, no significant correlations were observed between the subjective SNOT scores and olfactory function as assessed by the SST-12 [33].
Examining SNOT-20, we identified a subtle correlation trend between SNOT-20 and NPS (r = 0.17, p = 0.027). This observation aligns with analogous findings reported by a separate study, which also documented a weak trend (r = 0.33, p = 0.02) [34]. Conversely, other authors reached a divergent conclusion, that the correlation coefficient was nearly zero, suggesting a random relationship between SNOT-20 and endoscopy findings [35].
Focusing on subscores, our analysis unveiled significant correlations exclusively for the PNS subscore in SNOT-20 and the nasal subscore in the SNOT-22. These findings are consistent with previous literature, highlighting stronger correlations between polyp scores and nasal domains within the SNOT [36,37,38]. It’s suggested that the historically observed low correlations between endoscopic findings and PROMs may stem from the use of aggregate scores, thereby diluting meaningful correlations [37]. Thus, as might be intuitively expected, the NPS exhibits better predictive capacity for symptoms, particularly within nasal domains, when analyzed separately.
Correlation analyses of absolute changes over time
Jeong et al. conducted a 2022 meta-analysis, examining all previously used endoscopic nasal polyp scoring systems to assess their correlations with PROMs [19]. They employed the approach of correlating absolute changes over time, but still found no statistical significance. Our analysis yielded similar results, as statistically significant evidence was absent when correlating the absolute change in NPS with the absolute change in SNOT scores.
While a rapid initial response under dupilumab might suggest a monotonic trend, such a trend should still be detectable by Spearman’s rank correlation, which is sensitive to both linear and monotonic associations. Therefore, the lack of correlation in our study cannot be attributed to the quick response. It likely reflects variability in subjective symptom perception, which can obscure the relationship between NPS and SNOT scores.
Correlation analyses of relative changes
To the best of our knowledge, no other study has employed an approach similar to evaluating relative changes rather than absolute changes. Correlating the relative changes of PROMs with nasal polyp severity revealed significant results. Moreover, stratifying patients by baseline NPS revealed a significant correlation between NPS and relative SNOT score change in most groups (NPS 4, 6, 8) except for NPS 2 at baseline. These divergent results, whether considering absolute or relative values, can be explained by the fact that patients’ subjective perception of disease may differ greatly and patients with the same baseline NPS have a wide range of different baseline SNOT scores. Examining the relative differences in scores, rather than the absolute ones, reveals a significant percentage improvement in SNOT score with a concomitant percentage decrease in polyp score with dupilumab therapy, which represents a novel and clinically relevant finding. Opposing the conclusion that new nasal polyp grading systems with improved clinical utility are necessary because of their limited predictive capability [19], we argue that the lack of correlation between PROMs and NPS cannot be attributed to their limited reliability. Instead, we reemphasize that the subjective perception remains highly variable even at the same disease burden, making correlation analyses with relative changes stratified by baseline scores more appropriate. Introducing a new scoring system merely contributes to an expansion of scoring methods, potentially complicating cross-study comparisons when different measures are employed across various studies.
At this point, we also want to emphasize that it is unwarranted for insurance companies to consistently require absolute SNOT and polyp scores.
Strengths and limitations
A notable strength of our study is that it represents the first comprehensive attempt to employ relative and percentage measures in analyzing the correlations between objective scores and PROMs. However, some limitations should be considered. Participants were required to complete the SNOT questionnaire weekly, but inconsistent compliance may have impacted symptom assessment accuracy. The two questionnaires are essentially identical. The difference is that the SNOT-22 contains two additional questions: “Lack of good night’s sleep” and “Wake up tired”. In addition, one question differs between the two questionnaires: “Need to throat clearing” in the Snot-20 GAV and “Need to blow nose” in the SNOT-22.
Additionally, interpreting rhinoscopy findings for nasal polyp scoring can be challenging under certain conditions, such as severe mucosal swelling or limited patient cooperation. Changes in polyp size during therapy may lead to paradoxical shifts in polyp scores, i.e. revealing polyps between the middle turbinate and septum, allowing for potential bias. Furthermore, although we reviewed the videos in cases of uncertainty, a residual risk of inter- and intra-observer variability remains, as the assessment is inherently subject to the subjective judgment of the examiners. Previous surgery may also interfere with the NPS grading system, potentially introducing further biases.
Conclusion
Dupilumab therapy demonstrated substantial improvements in SNOT and NPS. However, our findings highlight the limitations of absolute correlation analyses, revealing only a weak trend for SNOT-20 and correlations only within the subscore analysis of the nasal subscores. The limitations of absolute correlation analysis are likely influenced by the inherent variability in subjective perception. Hence, it appears more suitable to correlate relative changes and to stratify patients based on their baseline values. Therefore, to ultimately enhance our understanding of CRSwNP treatment outcomes, future research should continue to explore the utility of relative change correlation analyses.
References
Kwah JH, Peters AT (2019) Nasal polyps and rhinosinusitis. Allergy Asthma Proc 40(6):380–384
Stevens WW, Schleimer RP, Kern RC (2016) Chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol Pract 4(4):565–572
Khan A, Huynh TMT, Vandeplas G, Joish VN, Mannent LP, Tomassen P et al (2019) The GALEN rhinosinusitis cohort: chronic rhinosinusitis with nasal polyps affects health-related quality of life. Rhinology 57(5):343–351
Bachert C, Bhattacharyya N, Desrosiers M, Khan AH (2021) Burden of disease in chronic rhinosinusitis with nasal polyps. J Asthma Allergy. :127–134
Laidlaw TM, Buchheit KM (2020) Biologics in chronic rhinosinusitis with nasal polyposis. Ann Allergy Asthma Immunol 124(4):326–332
Mullol J, Azar A, Buchheit KM, Hopkins C, Bernstein JA (2022) Chronic Rhinosinusitis with nasal polyps: quality of life in the Biologics era. J Allergy Clin Immunology: Pract 10(6):1434–53e9
Striz I, Golebski K, Strizova Z, Loukides S, Bakakos P, Hanania NA et al (2023) New insights into the pathophysiology and therapeutic targets of asthma and comorbid chronic rhinosinusitis with or without nasal polyposis. Clin Sci (Lond) 137(9):727–753
Hellings PW, Verhoeven E, Fokkens WJ (2021) State-of-the-art overview on biological treatment for CRSwNP. Rhinology 59(2):151–163
Fokkens WJ, Lund VJ, Hopkins C, Hellings PW, Kern R, Reitsma S et al (2020) European position paper on Rhinosinusitis and nasal polyps 2020. Rhinology 58(Suppl S29):1–464
Bachert C, Hellings PW, Mullol J, Hamilos DL, Gevaert P, Naclerio RM et al (2020) Dupilumab improves health-related quality of life in patients with chronic rhinosinusitis with nasal polyposis. Allergy 75(1):148–157
Hopkins C, Buchheit KM, Heffler E, Cohen NA, Olze H, Khan AH et al (2022) Improvement in Health-Related Quality of Life with Dupilumab in patients with moderate-to-severe asthma with Comorbid Chronic Rhinosinusitis with/without nasal polyps: an analysis of the QUEST study. J Asthma Allergy 15:767–773
Gevaert P, Omachi TA, Corren J, Mullol J, Han J, Lee SE et al (2020) Efficacy and safety of omalizumab in nasal polyposis: 2 randomized phase 3 trials. J Allergy Clin Immunol 146(3):595–605
Bachert C, Sousa AR, Lund VJ, Scadding GK, Gevaert P, Nasser S et al (2017) Reduced need for surgery in severe nasal polyposis with mepolizumab: randomized trial. J Allergy Clin Immunol 140(4):1024–1031e14
Gevaert P, Calus L, Van Zele T, Blomme K, De Ruyck N, Bauters W et al (2013) Omalizumab is effective in allergic and nonallergic patients with nasal polyps and asthma. J Allergy Clin Immunol 131(1):110–116e1
Ta NH, Gao J, Philpott C (eds) (2021) A systematic review to examine the relationship between objective and patient-reported outcome measures in sinonasal disorders: recommendations for use in research and clinical practice. International forum of allergy & rhinology
Rathi VK, Scangas GA, Metson RB, Xiao R, Nshuti L, Dusetzina SB (2022) Out-of-pocket costs of biologic treatments for chronic rhinosinusitis with nasal polyposis in the Medicare population. Int Forum Allergy Rhinology 12(10):1295–1298
Khosravi H, Zhang S, Anderson AM, Ferris LK, Choudhary S, Patton T (2020) Dupilumab drug survival, treatment failures, and insurance approval at a tertiary care center in the United States. J Am Acad Dermatol 82(4):1023–1024
Rudmik L, Hopkins C, Peters A, Smith TL, Schlosser RJ, Soler ZM (2015) Patient-reported outcome measures for adult chronic rhinosinusitis: a systematic review and quality assessment. J Allergy Clin Immunol 136(6):1532–40e2
Jeong SS, Chen T, Nguyen SA, Edwards TS, Schlosser RJ (2022) Correlation of polyp grading scales with patient symptom scores and olfaction in chronic rhinosinusitis: a systematic review and meta-analysis. Rhinology
Gevaert P, De Craemer J, Bachert C, Blauwblomme M, Chaker A, Cingi C et al (2023) European Academy of Allergy and Clinical Immunology position paper on endoscopic scoring of nasal polyposis. Allergy 78(4):912–922
Piccirillo JF, Merritt MG Jr, Richards ML (2002) Psychometric and clinimetric validity of the 20-item sino-nasal outcome test (SNOT-20). Otolaryngology—Head Neck Surg 126(1):41–47
Baumann I, Blumenstock G, DeMaddalena H, Piccirillo J, Plinkert P (2007) Lebensqualität Bei Patienten Mit Chronischer Rhinosinusitis. HNO 55(1):42–47
Usman AN (2017) Correlation between septal body and quality of life based on Sinonasal Outcome Test 20 (SNOT-20). J Med Sci 17(4):162–166
Khan AH, Reaney M, Guillemin I, Nelson L, Qin S, Kamat S et al (2022) Development of Sinonasal Outcome Test (SNOT-22) domains in chronic rhinosinusitis with nasal polyps. Laryngoscope 132(5):933–941
Toma S, Hopkins C (2016) Stratification of SNOT-22 scores into mild, moderate or severe and relationship with other subjective instruments. Rhinology 54(2):129–133
Team RC (2016) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www R-project org/
Agache I, Song Y, Alonso-Coello P, Vogel Y, Rocha C, Solà I et al (2021) Efficacy and safety of treatment with biologicals for severe chronic rhinosinusitis with nasal polyps: a systematic review for the EAACI guidelines. Allergy 76(8):2337–2353
Fujieda S, Matsune S, Takeno S, Asako M, Takeuchi M, Fujita H et al (2021) The Effect of Dupilumab on Intractable Chronic Rhinosinusitis with nasal polyps in Japan. Laryngoscope 131(6):E1770–e7
Hopkins C, Gillett S, Slack R, Lund VJ, Browne JP (2009) Psychometric validity of the 22-item Sinonasal Outcome Test. Clin Otolaryngol 34(5):447–454
Phillips KM, Hoehle LP, Caradonna DS, Gray ST, Sedaghat AR (2018) Minimal clinically important difference for the 22-item Sinonasal Outcome Test in medically managed patients with chronic rhinosinusitis. Clin Otolaryngol 43(5):1328–1334
Psaltis AJ, Li G, Vaezeafshar R, Cho K-S, Hwang PH (2014) Modification of the lund-kennedy endoscopic scoring system improves its reliability and correlation with patient-reported outcome measures. Laryngoscope 124(10):2216–2223
Misirovs R, Chan R, Lipworth B (2023) SNOT-5 and SNOT-22 relationship with endoscopic and radiological scores in chronic rhinosinusitis with nasal polyps. Annals of Allergy, Asthma & Immunology
Mauthe T, Meerwein CM, Ryser FS, Brà Hlmann C, Yalamanoglu A, Steiner UC et al (2024) Screening olfaction under dupilumab in chronic rhinosinusitis with nasal polyps. Rhinology 62(4):496–505
Schneider S, Campion NJ, Villazala-Merino S, Liu DT, Bartosik T, Landegger LD et al (2020) Associations between the quality of life and nasal polyp size in patients suffering from chronic rhinosinusitis without nasal polyps, with nasal polyps or aspirin-exacerbated respiratory disease. J Clin Med 9(4):925
Ryan WR, Ramachandra T, Hwang PH (2011) Correlations between symptoms, nasal endoscopy, and in-office computed tomography in post-surgical chronic rhinosinusitis patients. Laryngoscope 121(3):674–678
Dejaco D, Riedl D, Huber A, Moschen R, Giotakis AI, Bektic-Tadic L et al (2019) The SNOT-22 factorial structure in European patients with chronic rhinosinusitis: new clinical insights. Eur Arch Otorhinolaryngol 276(5):1355–1365
DeConde AS, Bodner TE, Mace JC, Alt JA, Rudmik L, Smith TL (2016) Development of a clinically relevant endoscopic grading system for chronic rhinosinusitis using canonical correlation analysis. Int Forum Allergy Rhinology 6(5):478–485
Sedaghat AR, Gray ST, Caradonna SD, Caradonna DS (2015) Clustering of chronic Rhinosinusitis Symptomatology reveals Novel associations with Objective Clinical and demographic characteristics. Am J Rhinol Allergy 29(2):100–105
Acknowledgements
None.
Funding
There was no financial support or funding for this project.
Open access funding provided by University of Zurich
Author information
Authors and Affiliations
Contributions
FSR, CB, and TM were engaged in the acquisition of data, particularly involving patient consultations. TM took charge of data extraction, manuscript composition, and contributed to statistical analysis. FSR conducted the statistical analysis and assisted with the manuscript preparation. CMM and AY gave crucial support throughout the project and participated in the editing process of the manuscript. MBS reviewed videos of patients with unclear polyp scores and was involved in patient consultations. MBS and UCS initiated the study’s conceptualization, offering valuable input during discussions, and critically reviewing the study. All authors read, edited, and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
MBS is a consultant for Sanofi, GSK, Novartis, Astra Zeneca, and MSD unrelated to this study. UCS is a consultant for Sanofi, GSK, TAKEDA, BioCryst unrelated to this study. All other researchers involved assure that there are no conflicts of interest.
Oxford centre for evidence-based medicine 2011
Level 3 of Evidence.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mauthe, T., Ryser, F.S., Brühlmann, C. et al. Correlation of sino-nasal outcome test and nasal polyp score in dupilumab-treated chronic rhinosinusitis with nasal polyps. Eur Arch Otorhinolaryngol (2024). https://doi.org/10.1007/s00405-024-08973-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00405-024-08973-7