Abstract
Objectives
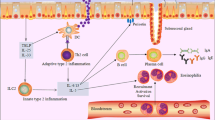
TAM receptors (TYRO3, AXL, and MER) play important roles in inflammatory responses, but their effects in chronic rhinosinusitis with nasal polyps (CRSwNP) remain elucidated. We aim to evaluate the values of TAM receptors in disease severity and postoperative recurrence of CRSwNP.
Methods
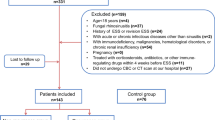
We initially enrolled 160 patients with CRSwNP who were treated with functional endoscopic sinus surgery (FESS) and postoperative recurrence was evaluated during the follow-up period. Circulating TAM receptor levels were detected by enzyme-linked immunosorbent assay (ELISA), and tissue expressions were measured by real-time polymerase chain reaction (RT-PCR) and immunohistochemical (IHC). The relationships between TAM receptor levels and postoperative recurrence were examined.
Results
A total of 150 patients completed the follow-up schedule, 49 patients experienced postoperative recurrence and the remaining 101 patients were non-recurrent. In recurrent CRSwNP patients, serum levels of TAM receptors were increased compared to those in non-recurrent patients and were positively correlated with disease severity scores (P < 0.05). Circulating TYRO3 and MER were identified as potential predictors of postoperative recurrence based on receiver operating characteristics (ROC) and Kaplan–Meier plots (P < 0.05). Furthermore, tissue TAM receptor levels, as determined by both RT-PCR and IHC, were enhanced in the recurrent group than in the non-recurrent group (P < 0.05) and were predictive of postoperative recurrence (P < 0.05). Interestingly, circulating TYRO3 and MER concentrations, as well as tissue TYRO3 expression, were found to be significantly increased in patients who experienced postoperative recurrence (P < 0.05). IHC images from the same patients revealed that TAM expressions were enhanced in the recurrent tissues compared to their baseline tissue levels.
Conclusions
Our laboratory results demonstrated that TAM receptors were increased in recurrent CRSwNP patients and associated with postoperative recurrence. Moreover, the new laboratory findings suggested that measuring circulating levels of TAM receptors might serve as a promising new approach to assess disease progression and predict the risk of postoperative recurrence.






Similar content being viewed by others
References
Orlandi RR, Kingdom TT, Smith TL et al (2021) International consensus statement on allergy and rhinology: rhinosinusitis 2021. Int Forum Allergy Rhinol 11(3):213–739. https://doi.org/10.1002/alr.22741
Abbas EE, Li C, Xie A et al (2021) Distinct clinical pathology and microbiota in chronic rhinosinusitis with nasal polyps endotypes. Laryngoscope 131(1):E34–E44. https://doi.org/10.1002/lary.28858
Klingler AI, Stevens WW, Tan BK et al (2021) Mechanisms and biomarkers of inflammatory endotypes in chronic rhinosinusitis without nasal polyps. J Allergy Clin Immunol 147(4):1306–1317. https://doi.org/10.1016/j.jaci.2020.11.037
Lou H, Zhang N, Bachert C et al (2018) Highlights of eosinophilic chronic rhinosinusitis with nasal polyps in definition, prognosis, and advancement. Int Forum Allergy Rhinol 8(11):1218–1225. https://doi.org/10.1002/alr.22214
Promsopa C, Kansara S, Citardi MJ et al (2016) Prevalence of confirmed asthma varies in chronic rhinosinusitis subtypes. Int Forum Allergy Rhinol 6(4):373–377. https://doi.org/10.1002/alr.21674
Geng B, Dilley M, Anterasian C (2021) Biologic therapies for allergic rhinitis and nasal polyposis. Curr Allergy Asthma Rep 21(6):36. https://doi.org/10.1007/s11882-021-01013-y
Gelardi M, Netti GS, Giancaspro R et al (2022) Chronic rhinosinusitis with nasal polyposis (CRSwNP): the correlation between expression of Galectin-10 and Clinical-Cytological Grading (CCG). Am J Rhinol Allergy 36(2):229–237. https://doi.org/10.1177/19458924211049867
Grayson JW, Hopkins C, Mori E et al (2020) Contemporary classification of chronic rhinosinusitis beyond polyps vs No polyps: a review. JAMA Otolaryngol Head Neck Surg 146(9):831–838. https://doi.org/10.1001/jamaoto.2020.1453
Bachert C, Marple B, Hosemann W et al (2020) Endotypes of chronic rhinosinusitis with nasal polyps: pathology and possible therapeutic implications. J Allergy Clin Immunol Pract 8(5):1514–1519. https://doi.org/10.1016/j.jaip.2020.03.007
Staudacher AG, Peters AT, Kato A et al (2020) Use of endotypes, phenotypes, and inflammatory markers to guide treatment decisions in chronic rhinosinusitis. Ann Allergy Asthma Immunol 124(4):318–325. https://doi.org/10.1016/j.anai.2020.01.013
Loftus CA, Soler ZM, Koochakzadeh S et al (2020) Revision surgery rates in chronic rhinosinusitis with nasal polyps: meta-analysis of risk factors. Int Forum Allergy Rhinol 10(2):199–207. https://doi.org/10.1002/alr.22487
Miglani A, Divekar RD, Azar A et al (2018) Revision endoscopic sinus surgery rates by chronic rhinosinusitis subtype. Int Forum Allergy Rhinol 8(9):1047–1051. https://doi.org/10.1002/alr.22146
Lee CH, Chun T (2019) Anti-inflammatory role of TAM family of receptor tyrosine kinases via modulating macrophage function. Mol Cells 42(1):1–7. https://doi.org/10.14348/molcells.2018.0419
Rothlin CV, Carrera-Silva EA, Bosurgi L et al (2015) TAM receptor signaling in immune homeostasis. Annu Rev Immunol 33:355–391. https://doi.org/10.1146/annurev-immunol-032414-112103
Vago JP, Amaral FA, van de Loo FAJ (2021) Resolving inflammation by TAM receptor activation. Pharmacol Ther 227:107893. https://doi.org/10.1016/j.pharmthera.2021.107893
Peeters MJW, Rahbech A, Thor Straten P (2020) TAM-ing T cells in the tumor microenvironment: implications for TAM receptor targeting. Cancer Immunol Immunother 69(2):237–244. https://doi.org/10.1007/s00262-019-02421-w
Boros E, Kellermayer Z, Balogh P et al (2018) Elevated expression of AXL May contribute to the epithelial-to-mesenchymal transition in inflammatory bowel disease patients. Mediators Inflamm 2018:3241406. https://doi.org/10.1155/2018/3241406
Grabiec AM, Hussell T (2016) The role of airway macrophages in apoptotic cell clearance following acute and chronic lung inflammation. Semin Immunopathol 38(4):409–423. https://doi.org/10.1007/s00281-016-0555-3
Kanazawa J, Masuko H, Yatagai Y et al (2019) Association analyses of eQTLs of the TYRO3 gene and allergic diseases in Japanese populations. Allergol Int 68(1):77–81. https://doi.org/10.1016/j.alit.2018.07.004
Fokkens WJ, Lund VJ, Mullol J et al (2012) EPOS 2012: European position paper on rhinosinusitis and nasal polyps 2012. A summary for otorhinolaryngologists. Rhinology 50(1):1–12. https://doi.org/10.4193/Rhino50E2
Zhang H, Xie S, Fan R et al (2022) Elevated ALCAM expression associated with endotypes and postoperative recurrence in chronic rhinosinusitis with nasal polyps. J Inflamm Res 15:1063–1077. https://doi.org/10.2147/jir.S350609
Lu PC, Lee TJ, Huang CC et al (2021) Serum eosinophil cationic protein: a prognostic factor for early postoperative recurrence of nasal polyps. Int Forum Allergy Rhinol 11(4):766–772. https://doi.org/10.1002/alr.22664
Li M, Xie Y, Zhao K et al (2021) Endoplasmic reticulum stress exacerbates inflammation in chronic rhinosinusitis with nasal polyps via the transcription factor XBP1. Clin Immunol 223:108659. https://doi.org/10.1016/j.clim.2020.108659
Alciato F, Sainaghi PP, Sola D et al (2010) TNF-alpha, IL-6, and IL-1 expression is inhibited by GAS6 in monocytes/macrophages. J Leukoc Biol 87(5):869–875. https://doi.org/10.1189/jlb.0909610
Ubil E, Caskey L, Holtzhausen A et al (2018) Tumor-secreted Pros1 inhibits macrophage M1 polarization to reduce antitumor immune response. J Clin Invest 128(6):2356–2369. https://doi.org/10.1172/JCI97354
Kim SY, Lim EJ, Yoon YS et al (2016) Liver X receptor and STAT1 cooperate downstream of Gas6/Mer to induce anti-inflammatory arginase 2 expression in macrophages. Sci Rep 6:29673. https://doi.org/10.1038/srep29673
Wu H, Zheng J, Xu S et al (2021) Mer regulates microglial/macrophage M1/M2 polarization and alleviates neuroinflammation following traumatic brain injury. J Neuroinflammation 18(1):2. https://doi.org/10.1186/s12974-020-02041-7
Chen SY, Chiang CF, Chiu KC et al (2019) Macrophage phenotypes and Gas6/Axl signaling in apical lesions. J Dent Sci 14(3):281–287. https://doi.org/10.1016/j.jds.2018.12.002
Saradna A, Do DC, Kumar S et al (2018) Macrophage polarization and allergic asthma. Transl Res 191:1–14. https://doi.org/10.1016/j.trsl.2017.09.002
Tsai ML, Tsai YG, Lin YC et al (2021) IL-25 induced ROS-mediated M2 macrophage polarization via AMPK-associated mitophagy. Int J Mol Sci. https://doi.org/10.3390/ijms23010003
Xia L, Wang X, Liu L et al (2021) lnc-BAZ2B promotes M2 macrophage activation and inflammation in children with asthma through stabilizing BAZ2B pre-mRNA. J Allergy Clin Immunol 147(3):921-32e9. https://doi.org/10.1016/j.jaci.2020.06.034
Tiotiu A, Zounemat Kermani N, Badi Y et al (2021) Sputum macrophage diversity and activation in asthma: Role of severity and inflammatory phenotype. Allergy 76(3):775–788. https://doi.org/10.1111/all.14535
Yao Y, Wang ZC, Liu JX et al (2017) Increased expression of TIPE2 in alternatively activated macrophages is associated with eosinophilic inflammation and disease severity in chronic rhinosinusitis with nasal polyps. Int Forum Allergy Rh 7(10):963–972. https://doi.org/10.1002/alr.21984
Bachert C, Han JK, Desrosiers MY et al (2022) Efficacy and safety of benralizumab in chronic rhinosinusitis with nasal polyps: A randomized, placebo-controlled trial. J Allergy Clin Immun 149(4):1309. https://doi.org/10.1016/j.jaci.2021.08.030
Loftus CA, Soler ZM, Desiato VM et al (2020) Factors impacting revision surgery in patients with chronic rhinosinusitis with nasal polyposis. Int Forum Allergy Rhinol 10(3):289–302. https://doi.org/10.1002/alr.22505
Wei B, Liu F, Zhang J et al (2018) Multivariate analysis of inflammatory endotypes in recurrent nasal polyposis in a Chinese population. Rhinology 56(3):216–226. https://doi.org/10.4193/Rhin17.240
Qi S, Yan B, Liu C et al (2020) Predictive significance of Charcot-Leyden Crystal mRNA levels in nasal brushing for nasal polyp recurrence. Rhinology 58(2):166–174. https://doi.org/10.4193/Rhin19.296
Rosati D, Rosato C, Pagliuca G et al (2020) Predictive markers of long-term recurrence in chronic rhinosinusitis with nasal polyps. Am J Otolaryngol 41(1):102286. https://doi.org/10.1016/j.amjoto.2019.102286
Loges S, Schmidt T, Tjwa M et al (2010) Malignant cells fuel tumor growth by educating infiltrating leukocytes to produce the mitogen Gas6. Blood 115(11):2264–2273. https://doi.org/10.1182/blood-2009-06-228684
Lou H, Huang Y, Chu X et al (2023) M2 macrophages upregulated by allergen exposure in seasonal allergic rhinitis. Int Arch Allergy Immunol. https://doi.org/10.1159/000529436
Shiratori H, Feinweber C, Luckhardt S et al (2017) THP-1 and human peripheral blood mononuclear cell-derived macrophages differ in their capacity to polarize in vitro. Mol Immunol 88:58–68. https://doi.org/10.1016/j.molimm.2017.05.027
Funding
This research was supported by the Natural Science Foundation of Hunan Province (2021JJ30754) and the Hunan Provincial Health Commission Scientific Research Project (202107010164).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest in preparing this article.
Ethical approval
This study was conducted following the recommendations of the Declaration of Helsinki. The Human Ethical Committee of the Affiliated Changsha Central Hospital, Hengyang Medical School of the University of South China approved this study (protocol: 2023005). Informed consent was obtained from the study participants before the study commencement.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
405_2024_8450_MOESM4_ESM.tif
Figure S1 The tissue mRNA levels of TAM receptors in CRSwNP patients without AR/AS. (A–C) When removed the patients with AR or AS, the tissue TAM receptor mRNA expressions were elevated in the recurrence group in comparison with the non-recurrence group. *P<0.05, ***P<0.001. CRSwNP, chronic rhinosinusitis with nasal polyps; AR, allergic rhinitis; AS, asthma
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhou, Y., Feng, Z., Wen, J. et al. Aberrant expressions of TAM receptors are associated with postoperative recurrence in chronic rhinosinusitis with nasal polyps. Eur Arch Otorhinolaryngol 281, 3005–3015 (2024). https://doi.org/10.1007/s00405-024-08450-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00405-024-08450-1