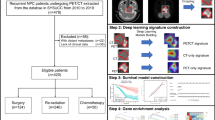
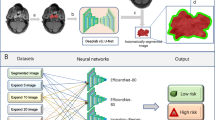
Abstract
Objective
As the prognosis of nasopharyngeal carcinoma (NPC) is influenced by various factors, making it difficult for clinical physicians to predict the outcome, the objective of this study was to develop a deep learning-based signature for risk stratification in NPC patients.
Methods
A total of 293 patients were enrolled in the study and divided into training, validation, and testing groups with a ratio of 7:1:2. MRI scans and corresponding clinical information were collected, and the 3-year disease-free survival (DFS) was chosen as the endpoint. The Res-Net18 algorithm was used to develop two deep learning (DL) models and another solely based on clinical characteristics developed by multivariate cox analysis. The performance of both models was evaluated using the area under the curve (AUC) and the concordance index (C-index). Discriminative performance was assessed using Kaplan–Meier survival analysis.
Results
The deep learning approach identified DL prognostic models. The MRI-based DL model showed significantly better performance compared to the traditional model solely based on clinical characteristics (AUC: 0.8861 vs 0.745, p = 0.04 and C-index: 0.865 vs 0.727, p = 0.03). The survival analysis showed significant survival differences between the risk groups identified by the MRI-based model.
Conclusion
Our study highlights the potential of MRI in predicting the prognosis of NPC through DL algorithm. This approach has the potential to become a novel tool for prognosis prediction and can help physicians to develop more valid treatment strategies in the future.






Similar content being viewed by others
Availability of data and materials
All patients’ data were collected from The First Affiliated Hospital of Xiamen University. We will not disclose the information of patients due to the need of privacy protection, but researchers could obtain relative data by contacting Chen Yang (yc924844040@foxmail.com).
References
Tang LL, Chen WQ, Xue WQ et al (2016) Global trends in incidence and mortality of nasopharyngeal carcinoma. Cancer Lett 374(1):22–30
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68(6):394–424
Chen YP, Chan ATC, Le QT, Blanchard P, Sun Y, Ma J (2019) Nasopharyngeal carcinoma. Lancet 394(10192):64–80
Pan JJ, Ng WT, Zong JF et al (2016) Prognostic nomogram for refining the prognostication of the proposed 8th edition of the AJCC/UICC staging system for nasopharyngeal cancer in the era of intensity-modulated radiotherapy. Cancer 122(21):3307–3315
Wong KCW, Hui EP, Lo KW et al (2021) Nasopharyngeal carcinoma: an evolving paradigm. Nat Rev Clin Oncol 18(11):679–695
Peng H, Dong D, Fang MJ et al (2019) Prognostic value of deep learning PET/CT-based radiomics: potential role for future individual induction chemotherapy in advanced nasopharyngeal carcinoma. Clin Cancer Res 25(14):4271–4279
Ng WT, Yuen KT, Au KH, Chan OS, Lee AW (2014) Staging of nasopharyngeal carcinoma–the past, the present and the future. Oral Oncol 50(6):549–554
Huang SH, O’sullivan B (2017) Overview of the 8th edition TNM Classification for Head and Neck Cancer. Curr Treat Options Oncol 18(7):40
Dong D, Fang MJ, Tang L et al (2020) Deep learning radiomic nomogram can predict the number of lymph node metastasis in locally advanced gastric cancer: an international multicenter study. Ann Oncol 31(7):912–920
Wang X, Li Q, Cai J et al (2020) Predicting the invasiveness of lung adenocarcinomas appearing as ground-glass nodule on CT scan using multi-task learning and deep radiomics. Transl Lung Cancer Res 9(4):1397–1406
Zhang L, Dong D, Zhang W et al (2020) A deep learning risk prediction model for overall survival in patients with gastric cancer: a multicenter study. Radiother Oncol 150:73–80
Daoud B, Morooka K, Kurazume R, Leila F, Mnejja W, Daoud J (2019) 3D segmentation of nasopharyngeal carcinoma from CT images using cascade deep learning. Comput Med Imaging Graph 77:101644
Liang S, Tang F, Huang X et al (2019) Deep-learning-based detection and segmentation of organs at risk in nasopharyngeal carcinoma computed tomographic images for radiotherapy planning. Eur Radiol 29(4):1961–1967
Lin L, Dou Q, Jin YM et al (2019) Deep learning for automated contouring of primary tumor volumes by MRI for nasopharyngeal carcinoma. Radiology 291(3):677–686
Zhang L, Wu X, Liu J et al (2021) MRI-based deep-learning model for distant metastasis-free survival in locoregionally advanced nasopharyngeal carcinoma. J Magn Reson Imaging 53(1):167–178
He KZX, Ren S et al (2016) Deep residual learning for image recognition. In: 2016 IEEE Conference on Computer Vision and Pattern Recognition (CVPR), IEEE
Vaswani ASN, Parmar N (2017) Attention is all you need. arXiv 2017
Paszke AGS, Massa F (2019) PyTorch: an imperative style, high-performance deep learning library. https://doi.org/10.48550/arXiv.1912.01703
Wu BXC, Dai X (2020) Visual transformers: token-based image representation and processing for computer vision. https://doi.org/10.48550/arXiv.2006.03677
Kingma DP, Ba J (2014) Adam: a method for stochastic optimization. https://arxiv.org/abs/1412.6980
An C, Li D, Li S et al (2022) Deep learning radiomics of dual-energy computed tomography for predicting lymph node metastases of pancreatic ductal adenocarcinoma. Eur J Nucl Med Mol Imaging 49(4):1187–1199
Hu Q, Wang G, Song X et al (2022) Machine learning based on MRI DWI radiomics features for prognostic prediction in nasopharyngeal carcinoma. Cancers (Basel) 14(13):3201
Li S, Deng YQ, Hua HL et al (2022) Deep learning for locally advanced nasopharyngeal carcinoma prognostication based on pre- and post-treatment MRI. Comput Methods Progr Biomed 219:106785
Qiang M, Li C, Sun Y et al (2021) A prognostic predictive system based on deep learning for locoregionally advanced nasopharyngeal carcinoma. J Natl Cancer Inst 113(5):606–615
Wang G, Mudgal P, Wang L et al (2021) TCR repertoire characteristics predict clinical response to adoptive CTL therapy against nasopharyngeal carcinoma. Oncoimmunology 10(1):1955545
Zhao X, Liang YJ, Zhang X et al (2022) Deep learning signatures reveal multiscale intratumor heterogeneity associated with biological functions and survival in recurrent nasopharyngeal carcinoma. Eur J Nucl Med Mol Imaging 49(8):2972–2982
Kim JY, Park JE, Jo Y et al (2019) Incorporating diffusion- and perfusion-weighted MRI into a radiomics model improves diagnostic performance for pseudoprogression in glioblastoma patients. Neuro Oncol 21(3):404–414
Li G, Li L, Li Y et al (2022) An MRI radiomics approach to predict survival and tumour-infiltrating macrophages in gliomas. Brain 145(3):1151–1161
Yu Y, He Z, Ouyang J et al (2021) Magnetic resonance imaging radiomics predicts preoperative axillary lymph node metastasis to support surgical decisions and is associated with tumor microenvironment in invasive breast cancer: a machine learning, multicenter study. EBioMedicine 69:103460
Zhang B, He X, Ouyang F et al (2017) Radiomic machine-learning classifiers for prognostic biomarkers of advanced nasopharyngeal carcinoma. Cancer Lett 403:21–27
Lambin P, Leijenaar RTH, Deist TM et al (2017) Radiomics: the bridge between medical imaging and personalized medicine. Nat Rev Clin Oncol 14(12):749–762
Mayerhoefer ME, Materka A, Langs G et al (2020) Introduction to radiomics. J Nucl Med 61(4):488–495
Yip SS, Aerts HJ (2016) Applications and limitations of radiomics. Phys Med Biol 61(13):R150-166
He K, Zhang X, Ren S, Sun J (2016) Deep residual learning for image recognition. Springer, pp 770–778
Khan H (2018) DM-L based feature extraction and classifier ensemble for object recognition. J Signal Inf Process 9:92–110
Dong D, Tang L, Li ZY et al (2019) Development and validation of an individualized nomogram to identify occult peritoneal metastasis in patients with advanced gastric cancer. Ann Oncol 30(3):431–438
Koshimizu H, Kojima R, Okuno Y (2020) Future possibilities for artificial intelligence in the practical management of hypertension. Hypertens Res 43(12):1327–1337
Schwendicke F, Samek W, Krois J (2020) Artificial intelligence in dentistry: chances and challenges. J Dent Res 99(7):769–774
Streich J, Romero J, Gazolla J et al (2020) Can exascale computing and explainable artificial intelligence applied to plant biology deliver on the United Nations sustainable development goals? Curr Opin Biotechnol 61:217–225
Taylor JET, Taylor GW (2021) Artificial cognition: How experimental psychology can help generate explainable artificial intelligence. Psychon Bull Rev 28(2):454–475
Chan KCA, Woo JKS, King A et al (2017) Analysis of plasma Epstein-Barr virus DNA to screen for nasopharyngeal cancer. N Engl J Med 377(6):513–522
Funding
This study was supported by the Natural Science Foundation of Fujian Province [Grant Nos. 2020J011220 and 2020J011236], the Key Medical and Health Projects in Xiamen (Grant No. 3502Z20209002), and the National Natural Science Foundation of China [Grant No. 81772893].
Author information
Authors and Affiliations
Contributions
CY, QL, and LSW conceived the idea. CY and YC designed the study. LCZ collected the data. YC finished the content analysis. QL and YC helped editing the article pictures. CY and YC drafted the manuscript. QL and LSW reviewed and corrected the manuscript. CY and YC have contributed equally to this work.
Corresponding authors
Ethics declarations
Conflict of interest
All authors claim that there are no potential conflicts of interest in the study.
Ethical approval and consent to participate
The study was approved by The First Affiliated Hospital of Xiamen University Ethical Review Committee (XMCTRC-2022-01), and informed consent was not required due to its retrospective nature.
Consent for publication
All authors have given their consent for publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Yang, C., Chen, Y., Zhu, L. et al. A deep learning MRI-based signature may provide risk-stratification strategies for nasopharyngeal carcinoma. Eur Arch Otorhinolaryngol 280, 5039–5047 (2023). https://doi.org/10.1007/s00405-023-08084-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00405-023-08084-9