Abstract
Purpose
The aim of this study was to analyze the hearing outcomes and quality of life in a series of 52 patients affected by conductive or mixed hearing loss and treated with Bonebridge®.
Methods
52 of 71 patients implanted with Bonebridge® between October 2012 and January 2022, were included in the study. We compared the air conduction thresholds at the frequencies 500, 1000, 2000, 3000, 4000 Hz, the SRT50% and the World Recognition Score at an intensity of 50 dB with and without the implant. The Abbreviated Profile of Hearing Aid Benefit (APHAB) was employed to assess the quality of life of patients.
Results
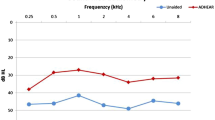
The liminal tone audiometry (free field) pure tone average for air conduction after 6 months with the implant was 35.12 dB, obtaining a mean gain of 31.83 dB. With Bonebridge®, the mean SRT was 34.17 dB, whereas before the surgery no patient achieved 50% of correct answers at a sound intensity of 50 dB. The world recognition score at 50 dB changed from 11% without the implant to 85% with it. We observed one case of implant failure and one case of implant exposure. The APHAB questionnaire showed an improvement after implantation in practically all the subscales.
Conclusions
The hearing outcomes and the subjective benefits reported by patients obtained in our study are similar to those published in the literature. Bonebridge® represents an excellent method for the rehabilitation of patients with conductive and mixed hearing loss, showing a low rate of complications.





Similar content being viewed by others
References
Stenfelt S (2011) Acoustic and physiologic aspects of bone conduction hearing. Adv Otorhinolaryngol 71:10–21
Tjellström A, Rosenhall U, Lindstrom J, Hallen O, Albrektsson T, Branemark I (1983) Five-year experience with skin-penetrating bone-anchored implants in the temporal bone. Acta Otolaryngol 95:568–575
Carnevale C, Til-Pérez G, Arancibia-Tagle DJ, Tomás-Barberán MD, Sarría-Echegaray PL (2019) Hearing outcomes of the active bone conduction system Bonebridge ® in conductive or mixed hearing loss. Acta Otorrinolaringol Esp 70(2):80–88
Magele A, Schoerg P, Stanek B, Gradl B, Sprinzl GM (2019) Active transcutaneous bone conduction hearing implants: systematic review and meta-analysis. PLoS ONE. https://doi.org/10.1371/journal.pone.0221484
Sprinzl GM, Wolf-Magele A (2016) The bonebridge bone conduction hearing implant: Indication criteria, surgery and a systematic review of the literature. Clin Otolaryngol 41:131–143
Sprinzl G, Lenarz T, Ernst A, Hagen R (2013) First European Multicenter results with a new transcutaneous bone conduction hearing implant system: short term safety and efficacy. Otol Neurotol 34:1076–1083
Manrique M, Sanhueza I, Manrique R, de Abajo J (2014) A new bone conduction implant: surgical technique and results. Otol Neurotol 35:216–220
Cywka KB, Skarzynski H, Król B, Skarzynski PH (2021) The Bonebridge BCI 602 active transcutaneous bone conduction implant in children: objective and subjective benefits. J Clin Med 10(24):5916. https://doi.org/10.3390/jcm10245916
Plontke SK, Götze G, Wenzel C, Rahne T, Mlynski R (2020) Implantation of a new active bone conduction hearing device 395 with optimized geometry. HNO 68:106–115. https://doi.org/10.1007/s00106-020-00877-2.396
Utrilla C, Gavilán J, García-Raya P, Calvino M, Lassaletta L (2020) MRI after Bonebridge implantation: a comparison of two 397 implant generations. Eur Arch Otorhinolaryngol 278:3203–3209. https://doi.org/10.1007/s00405-020-06380-2
Carnevale C, Til-Pérez G, Arancibia-Tagle D, Tomás-Barberán M, Sarría-Echegaray P (2021) Cervicofacial surgery and implantable hearing device extrusion: management of challenging cases. J Laryngol Otol 135(3):212–216
Rivas JA, Rincón LA, Garcia L, Rivas A, Tamayo C, Forero VH (2013) Implantes auditivos de conducción ósea percutáneo, transcutáneo: comparación. Acta Otorrinolaringol Cir Cabeza Cuello 41:17–24
Skarżyński PH, Ratuszniak A, Król B, Kozieł M, Osińska K, Cywka KB, Sztabnicka A, Skarżyński H (2019) The Bonebridge in adults with mixed and conductive hearing loss: audiological and quality of life outcomes. Audiol Neurotol 24:90–99
Seiwerth I, Fröhlich L, Schilde S, Götze G, Plontke SK, Rahne T (2022) Clinical and functional results after implantation of the bonebridge, a semi-implantable, active transcutaneous bone conduction device, in children and adults. Eur Arch Otorhinolaryngol 279(1):101–113
Baumgartner WD, Hamzavi JS, Boheim K, Wolf-Magele A, Schlogel M, Riechelmann H, Zorowka P, Koci V, Keck T, Potzinger P, Sprinzl G (2016) A new transcutaneous bone conduction hearing implant: short-term safety and efficacy in children. Otol Neurotol 37(6):713–720. https://doi.org/10.1097/MAO.0000000000001038
Schmerber S, Deguine O, Marx M, Van de Heyning P, Sterkers O, Mosnier I, Garin P, Godey B, Vincent C, Venail F, Mondain M, Deveze A, Lavieille JP, Karkas A (2017) Safety and effectiveness of the Bonebridge transcutaneous active direct-drive bone-conduction hearing implant at 1-year device use. Eur Arch Otorhinolaryngol 274(4):1835–1851
Garciera M, Lavedrinea A, Gagneuxa C, Eluecquec T, Grayeli AB (2021) Bone-anchored and closed skin Bonebridge implant in adults: hearing performances and quality of life. Audiol Neurootol 26(5):310–316. https://doi.org/10.1159/000512496
Barbara M, Perotti M, Gioia B, Volpini L, Monini S (2013) Transcutaneous bone-conduction hearing device: audiological and surgical aspects in a first series of patients with mixed hearing loss. Acta Otolaryngol 133:1058–1064
Zernotti ME, di Gregorio MF, Galeazzi P, Tabernero P (2016) Comparative outcomes of active and passive hearing devices by transcutaneous bone conduction. Acta Otolaryngol 136:556–558
Lassaletta L, Sanchez-Cuadrado I, Muñoz E, Gavilan J (2014) Retrosigmoid implantation of an active bone conduction stimulator in a patient with chronic otitis media. Auris Nasus Larynx 41:84–87
Zernotti ME, Sarasty AB (2015) Active bone conduction prosthesis: Bonebridge (TM). Int Arch Otorhinolaryngol 19:343–348
Carnevale C, Tomás-Barberán M, Til-Pérez G, Sarría-Echegaray P (2019) The Bonebridge active bone conduction system: a fast and safe technique for a middle fossa approach. J Laryngol Otol 133(4):344–347
Plontke SK, Götze G, Wenzel C, Rahne T, Mlynski R (2020) Implantation of a new active bone conduction hearing device with optimized geometry. HNO 68:106–115
You P, Siegel LH, Kassam Z, Hebb M, Parnes L, Ladak HM, Agrawal SK (2019) The middle fossa approach with self-drilling screws: a novel technique for BONEBRIDGE implantation. J Otolaryngol Head Neck Surg 48:1–10
Lassaletta L, Calvino M, Zernotti M, Gavilan J (2016) Postoperative pain in patients undergoing a transcutaneous active bone conduction implant (Bonebridge). Eur Arch Otorhinolaryngol 273(12):4103–4110
Law EK, Bhatia KS, Tsang WS, Tong MC, Shi L (2016) CT pre-operative planning of a new semi-implantable bone conduction hearing device. Eur Radiol 26(6):1686–1695. https://doi.org/10.1007/s00330-015-3983-x
Bianchin G, Bonali M, Russo M, Tribi L (2015) Active bone conduction system: outcomes with the Bonebridge transcutaneous device. ORL J Otorhinolaryngol Relat Spec 77(1):17–26. https://doi.org/10.1159/000371425
Weiss R, Leinung M, Baumann U, Weissgerber T, Rader T, Stover T (2017) Improvement of speech perception in quiet and in noise without decreasing localization abilities with the bone conduction device Bonebridge. Eur Arch Otorhinolaryngol 274(5):2107–2115. https://doi.org/10.1007/s00405-016-4434-2
Wimmer W, Gerber N, Guignard J, Dubach P, Kompis M, Weber S, Caversaccio M (2015) Topographic bone thickness maps for Bonebridge implantations. Eur Arch Otorhinolaryngol 272(7):1651–1658. https://doi.org/10.1007/s00405-014-2976-8
David T, Ramsden JD, Gordon KA, James AL, Papsin BC (2009) Soft tissue com- plications after small incision pediatric cochlear implantation. Laryngoscope 119:980–983
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
All authors have provided substantial contributions to the conception or design of the work or the interpretation of data for the work. All of them worked on the draft or revised it critically for important intellectual content. The final version was approved for publishing by all authors. The authors agree on accountability for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
The work was approved by ethics committee of Son Espases University Hospital and was performed in accordance with the ethical standards.
Consent for publication
All authors give have seen this manuscript and agree with its contents for publication.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Carnevale, C., Morales-Olavarría, C., Til-Pérez, G. et al. Bonebridge® bone conduction implant. Hearing outcomes and quality of life in patients with conductive/mixed hearing loss. Eur Arch Otorhinolaryngol 280, 1611–1619 (2023). https://doi.org/10.1007/s00405-022-07631-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00405-022-07631-0