Abstract
Purpose
GSP301 is a fixed-dose combination of olopatadine hydrochloride (antihistamine) and mometasone furoate (corticosteroid). This meta-analysis aims to evaluate the efficacy and safety of GSP301 in the treatment of allergic rhinitis.
Methods
A systematic review and meta-analysis were conducted. The data were collected from PubMed, Cochrane Central Register of Controlled Trials and Embase databases till June 2021. In patients with AR, short-term (2/6 weeks) and long-term (52 weeks) effects of GSP301 were assessed. Average morning and evening 12-h reflective total nasal symptom score (rTNSS), instantaneous total nasal symptom score (iTNSS), reflective total ocular symptom score (rTOSS), instantaneous total ocular symptom score(iTOSS), Physician-assessed nasal symptom score (PNSS), rhinoconjunctivitis quality of life (RQLQ), rhinitis control assessment test (RCAT) and adverse events (AEs) were measured.
Results
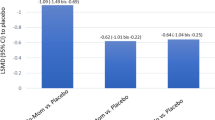
Five randomized controlled trials were included. GSP301 showed greatly improvement in rTNSS (MD = – 0.99; [95% CI – 1.19 to – 0.79]; P < 0.01; I2 = 0), iTNSS (MD = – 1.05; [95% CI – 1.44 to – 0.67]; P < 0.01; I2 > 50%), rTOSS (MD = – 0.50; [95% CI – 0.72 to – 0.29]; P < 0.01; I2 = 0), iTOSS (MD = – 0.64; [95% CI – 1.02 to – 0.26]; P < 0.01; I2 > 50%), PNSS (MD = – 1.01; [95% CI – 1.32 to – 0.69]; P < 0.01; I2 = 22.13%), RQLQ (MD = – 0.43; [95% CI – 0.57 to – 0.30]; P < 0.01; I2 = 0%) and RCAT (MD = 1.94; [95% CI 1.43–2.45]; P < 0.01; I2 = 0%) in the short term. No statistical difference was observed in the outcome of long-term PNSS, RQLQ and RCAT.
Conclusion
GSP301 is a safe and well-tolerated medication. It showed short-term benefits for seasonal and perennial AR, but may not help to improve patients’ quality of life and rhinitis control in the long run.













Similar content being viewed by others
References
Bernstein DI, Schwartz G, Bernstein JA (2016) Allergic rhinitis: mechanisms and treatment. Immunol Allergy Clin North Am 36(2):261–278
Dykewicz MS, Wallace DV, Baroody F et al (2017) Treatment of seasonal allergic rhinitis: an evidence-based focused 2017 guideline update. Ann Allergy Asthma Immunol 119(6):489-511.e441
Greiner AN, Hellings PW, Rotiroti G, Scadding GK (2011) Allergic rhinitis. Lancet 378(9809):2112–2122
Marple BF, Fornadley JA, Patel AA et al (2007) Keys to successful management of patients with allergic rhinitis: focus on patient confidence, compliance, and satisfaction. Otolaryngol Head Neck Surg 136(6 Suppl):S107-124
Meltzer EO (2016) Allergic rhinitis: burden of illness, quality of life, comorbidities, and control. Immunol Allergy Clin N Am 36(2):235–248
Meltzer EO, Blaiss MS, Naclerio RM et al (2012) Burden of allergic rhinitis: allergies in America, Latin America, and Asia-Pacific adult surveys. Allergy Asthma Proc 33(Suppl 1):S113-141
Frew AJ (2003) 25. Immunotherapy of allergic disease. J Allergy Clin Immunol 111(2 Suppl):S712-719
Bangalore S, Kamalakkannan G, Parkar S, Messerli FH (2007) Fixed-dose combinations improve medication compliance: a meta-analysis. Am J Med 120(8):713–719
Harrow B, Sedaghat AR, Caldwell-Tarr A, Dufour R (2016) A comparison of health care resource utilization and costs for patients with allergic rhinitis on single-product or free-combination therapy of intranasal steroids and intranasal antihistamines. J Manag Care Spec Pharm 22(12):1426–1436
Prenner BM (2016) A review of the clinical efficacy and safety of MP-AzeFlu, a novel intranasal formulation of azelastine hydrochloride and fluticasone propionate, in clinical studies conducted during different allergy seasons in the US. J Asthma Allergy 9:135–143
Price D, Shah S, Bhatia S et al (2013) A new therapy (MP29-02) is effective for the long-term treatment of chronic rhinitis. J Investig Allergol Clin Immunol 23(7):495–503
Brożek JL, Bousquet J, Agache I et al (2017) Allergic Rhinitis and its Impact on Asthma (ARIA) guidelines-2016 revision. J Allergy Clin Immunol 140(4):950–958
Fairchild CJ, Meltzer EO, Roland PS, Wells D, Drake M, Wall GM (2007) Comprehensive report of the efficacy, safety, quality of life, and work impact of Olopatadine 0.6% and Olopatadine 0.4% treatment in patients with seasonal allergic rhinitis. Allergy Asthma Proc 28(6):716–723
Meltzer EO, Hampel FC, Ratner PH et al (2005) Safety and efficacy of olopatadine hydrochloride nasal spray for the treatment of seasonal allergic rhinitis. Ann Allergy Asthma Immunol 95(6):600–606
Patel D, Garadi R, Brubaker M et al (2007) Onset and duration of action of nasal sprays in seasonal allergic rhinitis patients: olopatadine hydrochloride versus mometasone furoate monohydrate. Allergy Asthma Proc 28(5):592–599
Penagos M, Compalati E, Tarantini F, Baena-Cagnani CE, Passalacqua G, Canonica GW (2008) Efficacy of mometasone furoate nasal spray in the treatment of allergic rhinitis. Meta-analysis of randomized, double-blind, placebo-controlled, clinical trials. Allergy 63(10):1280–1291
Higgins JP, Altman DG, Gøtzsche PC et al (2011) The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 343:d5928
Segall N, Prenner B, Lumry W, Caracta CF, Tantry SK (2019) Long-term safety and efficacy of olopatadine-mometasone combination nasal spray in patients with perennial allergic rhinitis. Allergy Asthma Proc 40(5):301–310
Andrews CP, Mohar D, Salhi Y, Tantry SK (2020) Efficacy and safety of twice-daily and once-daily olopatadine-mometasone combination nasal spray for seasonal allergic rhinitis. Ann Allergy Asthma Immunol 124(2):171-178.e172
Gross GN, Berman G, Amar NJ, Caracta CF, Tantry SK (2019) Efficacy and safety of olopatadine-mometasone combination nasal spray for the treatment of seasonal allergic rhinitis. Ann Allergy Asthma Immunol 122(6):630-638.e633
Hampel FC, Pedinoff AJ, Jacobs RL, Caracta CF, Tantry SK (2019) Olopatadine-mometasone combination nasal spray: Evaluation of efficacy and safety in patients with seasonal allergic rhinitis. Allergy Asthma Proc 40(4):261–272
Patel P, Salapatek AM, Tantry SK (2019) Effect of olopatadine-mometasone combination nasal spray on seasonal allergic rhinitis symptoms in an environmental exposure chamber study. Ann Allergy Asthma Immunol 122(2):160-166.e161
Cook CE (2008) Clinimetrics corner: the minimal clinically important change score (MCID): a necessary pretense. J Man Manip Ther 16(4):E82-83
Barnes ML, Vaidyanathan S, Williamson PA, Lipworth BJ (2010) The minimal clinically important difference in allergic rhinitis. Clin Exp Allergy 40(2):242–250
Baroody FM, Naclerio RM (2011) Nasal-ocular reflexes and their role in the management of allergic rhinoconjunctivitis with intranasal steroids. World Allergy Organ J 4(1 Suppl):S1-5
Juniper EF, Guyatt GH, Griffith LE, Ferrie PJ (1996) Interpretation of rhinoconjunctivitis quality of life questionnaire data. J Allergy Clin Immunol 98(4):843–845
Patel P, Salapatek AM, Talluri RS, Tantry SK (2018) Pharmacokinetics of intranasal mometasone in the fixed-dose combination GSP301 versus two monotherapy intranasal mometasone formulations. Allergy Asthma Proc 39(3):232–239
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors declare that they do not have any conflict of interest.
Ethical approval
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chen, R., Zheng, D., zhang, Y. et al. Efficacy and safety of twice-daily olopatadine–mometasone combination nasal spray (GSP301) in the treatment of allergic rhinitis: a systematic review and meta-analysis. Eur Arch Otorhinolaryngol 279, 1691–1699 (2022). https://doi.org/10.1007/s00405-021-07085-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00405-021-07085-w