Abstract
Purpose
Nasopharyngeal carcinoma (NPC) is a malignant tumor endangering human health. Gemcitabine or cisplatin chemotherapy has been regarded as effective treatment for patients with locoregionally advanced NPC. However, the effect of gemcitabine plus cisplatin concurrent chemoradiotherapy (CCRT) remained controversial among the studies. Therefore, we conducted this meta-analysis to assess the efficacy and safety of induction chemotherapy by gemcitabine and cisplatin (GP regimen) in patients with locoregionally advanced NPC.
Methods
A systematic literature search was performed using PubMed, Web of Science, and Embase to evaluate the survival benefit and toxicity profiles of patients with locoregionally advanced NPC who were treated with CCRT. A random-effects model or a fixed-effects model was used to pool the data according to the heterogeneity among the included studies.
Results
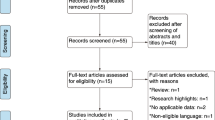
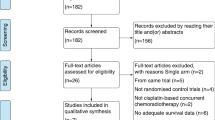
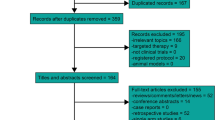
A total of five studies with 1286 patients met the inclusion criteria and were included in this meta-analysis. Pooled estimate showed that GP regimen was associated with significant improvements in OS (HR = 0.57, 95% CI 0.45, 0.73; P < 0.001), DFS (HR = 0.56, 95% CI 0.47, 0.66; P < 0.001), and DRFS (HR = 0.51, 95% CI 0.36, 0.73; P < 0.001), but not in LRFS (HR = 0.54, 95% CI 0.25, 1.19; P = 0.126) and ORR (RR = 1.30, 95% CI 0.54, 3.09; P = 0.556). Moreover, the incidence of adverse events of all grades (RR = 1.15, 95%CI 0.11, 1.38; P = 0.063) or grade 3–4 (RR = 0.96, 95%CI 0.57, 1.29; P = 0.385), was comparable between GP regimen and control treatments.
Conclusion
Our meta-analysis indicated that the patients with locoregionally advanced NPC could benefit from the regimen of gemcitabine plus cisplatin induction chemotherapy.





Similar content being viewed by others
Availability of data and material
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68(6):394–424. https://doi.org/10.3322/caac.21492
Mao YP, Xie FY, Liu LZ, Sun Y, Li L, Tang LL et al. Re-evaluation of 6th edition of AJCC staging system for nasopharyngeal carcinoma and proposed improvement based on magnetic resonance imaging. Int J Radiat Oncol Biol Phys. 2009;73(5):1326–1334. https://doi.org/10.1016/j.ijrobp.2008.07.062.
Wei WI, Sham JS (2005) Nasopharyngeal carcinoma. Lancet 365(9476):2041–2054. https://doi.org/10.1016/s0140-6736(05)66698-6
Garden AS, Harris J, Vokes EE, Forastiere AA, Ridge JA, Jones C et al (2004) Preliminary results of radiation therapy oncology Group 97–03: a randomized phase ii trial of concurrent radiation and chemotherapy for advanced squamous cell carcinomas of the head and neck. J Clin Oncol 22(14):2856–2864. https://doi.org/10.1200/jco.2004.12.012
Xu J, He X, Cheng K, Guo W, Bian X, Jiang X et al (2014) Concurrent chemoradiotherapy with nedaplatin plus paclitaxel or fluorouracil for locoregionally advanced nasopharyngeal carcinoma: Survival and toxicity. Head Neck 36(10):1474–1480. https://doi.org/10.1002/hed.23487
Mi JL, Zhang B, Pan YF, Su YX, Fan JF, Liao SF et al (2017) Chemotherapy regimens containing taxanes or fluorouracil in nasopharyngeal carcinoma: Which better? Oral Oncol 74:34–39. https://doi.org/10.1016/j.oraloncology.2017.09.003
Chen X, Hong Y, Feng J, Ye J, Zheng P, Guan X et al (2014) Concurrent chemoradiotherapy comparison of taxanes and platinum versus 5-fluorouracil and platinum in nasopharyngeal carcinoma treatment. Chin Med J (Engl) 127(1):142–149
He Y, Guo T, Wang J, Sun Y, Guan H, Wu S et al (2019) Which induction chemotherapy regimen followed by cisplatin-based concurrent chemoradiotherapy is the best choice among PF, TP and TPF for locoregionally advanced nasopharyngeal carcinoma? Ann Transl Med 7(5):104. https://doi.org/10.21037/atm.2019.02.15
Stevens CW, Lee JS, Cox J, Komaki R (2000) Novel approaches to locally advanced unresectable non-small cell lung cancer. Radiother Oncol 55(1):11–18. https://doi.org/10.1016/s0167-8140(00)00163-8
Viani GA, Afonso SL, Tavares VC, da Silva LG, Stefano EJ (2011) Weekly gemcitabine and cisplatin in combination with radiotherapy in patients with locally advanced head-and-neck cancer: Phase I study. Int J Radiat Oncol Biol Phys 81(4):e231–e235. https://doi.org/10.1016/j.ijrobp.2011.02.012
Bergman AM, Ruiz van Haperen VW, Veerman G, Kuiper CM, Peters GJ. Synergistic interaction between cisplatin and gemcitabine in vitro. Clin Cancer Res. 1996;2(3):521–530
Moher D, Liberati A, Tetzlaff J, Altman DG (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 339:b2535. https://doi.org/10.1136/bmj.b2535
Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD et al (2011) The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 343:d5928. https://doi.org/10.1136/bmj.d5928
Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ 327(7414):557–560. https://doi.org/10.1136/bmj.327.7414.557
DerSimonian R, Laird N (1986) Meta-analysis in clinical trials. Control Clin Trials 7(3):177–188
Mantel N, Haenszel W (1959) Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst 22(4):719–748
Begg CB, Mazumdar M (1994) Operating characteristics of a rank correlation test for publication bias. Biometrics 50(4):1088–1101
Egger M, Davey Smith G, Schneider M, Minder C (1997) Bias in meta-analysis detected by a simple, graphical test. BMJ 315(7109):629–634
Zhang Y, Chen L, Hu GQ, Zhang N, Zhu XD, Yang KY et al (2019) Gemcitabine and cisplatin induction chemotherapy in nasopharyngeal carcinoma. N Engl J Med 381(12):1124–1135. https://doi.org/10.1056/NEJMoa1905287
Kong XY, Lu JX, Yu XW, Zhang J, Xu QL, Zhang RJ et al (2019) Gemcitabine plus cisplatin versus fluorouracil plus cisplatin as a first-line concurrent chemotherapy regimen in nasopharyngeal carcinoma: a prospective, multi-institution, randomized controlled phase II study. Cancer Chemother Pharmacol 84(1):155–161. https://doi.org/10.1007/s00280-019-03858-7
Tan T, Lim WT, Fong KW, Cheah SL, Soong YL, Ang MK et al (2015) Concurrent chemo-radiation with or without induction gemcitabine, Carboplatin, and Paclitaxel: a randomized, phase 2/3 trial in locally advanced nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys 91(5):952–960. https://doi.org/10.1016/j.ijrobp.2015.01.002
Zhang L, Huang Y, Hong S, Yang Y, Yu G, Jia J et al (2016) Gemcitabine plus cisplatin versus fluorouracil plus cisplatin in recurrent or metastatic nasopharyngeal carcinoma: a multicentre, randomised, open-label, phase 3 trial. Lancet 388(10054):1883–1892. https://doi.org/10.1016/s0140-6736(16)31388-5
Zang J, Xu M, Li C, Zhao L, Luo S, Wang J et al (2020) Gemcitabine and cisplatin versus docetaxel and cisplatin as induction chemotherapy followed by concurrent chemoradiotherapy in locoregionally advanced nasopharyngeal carcinoma from non-endemic area of China. J Cancer Res Clin Oncol 146(9):2369–2378. https://doi.org/10.1007/s00432-020-03229-3
Sun Y, Li WF, Chen NY, Zhang N, Hu GQ, Xie FY et al (2016) Induction chemotherapy plus concurrent chemoradiotherapy versus concurrent chemoradiotherapy alone in locoregionally advanced nasopharyngeal carcinoma: a phase 3, multicentre, randomised controlled trial. Lancet Oncol 17(11):1509–1520. https://doi.org/10.1016/s1470-2045(16)30410-7
Liu T, Sun Q, Chen J, Wang F, Li B, Qin W et al (2018) A comparison of neoadjuvant chemotherapy with gemcitabine versus docetaxel plus cisplatin in locoregionally advanced nasopharyngeal carcinoma: a propensity score matching analysis. Cancer Manag Res 10:6237–6245. https://doi.org/10.2147/cmar.s186233
Zheng L, Liao W, Xu P, Li B, Wen H, Zhang S (2018) Tumor volume reduction after gemcitabine plus cisplatin induction chemotherapy in locally advanced nasopharyngeal cancer: comparison with paclitaxel and Cisplatin regimens. Med Sci Monit 24:8001–8008. https://doi.org/10.12659/msm.909736
Wildeman MA, Novalic Z, Verkuijlen SA, Juwana H, Huitema AD, Tan IB et al (2012) Cytolytic virus activation therapy for Epstein-Barr virus-driven tumors. Clin Cancer Res 18(18):5061–5070. https://doi.org/10.1158/1078-0432.ccr-12-0574
Lee N, Xia P, Quivey JM, Sultanem K, Poon I, Akazawa C et al (2002) Intensity-modulated radiotherapy in the treatment of nasopharyngeal carcinoma: an update of the UCSF experience. Int J Radiat Oncol Biol Phys 53(1):12–22. https://doi.org/10.1016/s0360-3016(02)02724-4
Wolden SL, Chen WC, Pfister DG, Kraus DH, Berry SL, Zelefsky MJ (2006) Intensity-modulated radiation therapy (IMRT) for nasopharynx cancer: update of the Memorial Sloan-Kettering experience. Int J Radiat Oncol Biol Phys 64(1):57–62. https://doi.org/10.1016/j.ijrobp.2005.03.057
Ouyang PY, Su Z, Mao YP, Liang XX, Liu Q, Deng W et al (2013) Prognostic impact of cigarette smoking on the survival of patients with established nasopharyngeal carcinoma. Cancer Epidemiol Biomarkers Prev 22(12):2285–2294. https://doi.org/10.1158/1055-9965.epi-13-0546
Leung SF, Chan AT, Zee B, Ma B, Chan LY, Johnson PJ et al (2003) Pretherapy quantitative measurement of circulating Epstein-Barr virus DNA is predictive of posttherapy distant failure in patients with early-stage nasopharyngeal carcinoma of undifferentiated type. Cancer 98(2):288–291. https://doi.org/10.1002/cncr.11496
Acknowledgements
None.
Funding
None.
Author information
Authors and Affiliations
Contributions
Lifeng Xiao and Yuru Li contributed to the study conception and design. All authors collected the data and performed the data analysis. All authors contributed to the interpretation of the data and the completion of figures and tables. All authors contributed to the drafting of the article and final approval of the submitted version.
Corresponding author
Ethics declarations
Conflict of interests
All the authors declare that they have no conflict of interest.
Code availability
Not applicable.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Xiao, L., Kang, W., Liao, J. et al. A meta-analysis comparing the efficacy and safety of gemcitabine plus cisplatin induction chemotherapy in patients with locoregionally advanced NPC. Eur Arch Otorhinolaryngol 279, 2441–2450 (2022). https://doi.org/10.1007/s00405-021-07033-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00405-021-07033-8