Abstract
Background
Previous studies reported that ferroptosis-related genes can regulate the process of tumor cell changes by regulating iron metabolism. However, the prognostic value of ferroptosis-related genes in LC remains to be further elucidated.
Methods
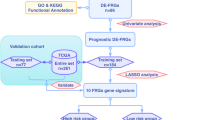
Ferroptosis-related gene expression profiles of coexisting ferroptosis-related genes were extracted from both cohorts (TCGA and GSE27020) for eligible analysis. LASSO Cox regression was utilized to build an optimum ferroptosis-related prognostic model. Kaplan–Meier curve was performed by log‐rank test, and time‐dependent ROC curve was constructed to evaluate the predictive power of this signature in both cohorts. GO and KEGG enrichment analysis was used to investigate the potential mechanism of differential enrichment signal pathways.
Results
112 LC patients from the TCGA cohort and 108 LC patients with clinical information from the GEO cohorts were eventually included in the study. Three ferroptosis-related genes were identified as an independent risk factor to establish the prognostic risk score. Kaplan–Meier curve represented that patients with high-risk group favors with worse OS than their low-risk group (P = 0.04). The good performance of the gene signature for predicting OS was evaluated by area under the curve (AUC) of time-dependent ROC curves achieved 0.74 at 3 years, and 0.70 at 5 years. Similar performance has been proved in the external validation cohort. GO and KEGG enrichment analysis have been performed to explore the signaling pathways and underlying mechanisms were significantly active in LC patients.
Conclusion
In summary, our study developed a ferroptosis-related model that could be an effective biomarker to predict the prognosis of laryngeal cancer.





Similar content being viewed by others
Data availability
The data used to support the findings of this study are included within the article.
References
Wu Y, Zhang Y, Zheng X, Dai F, Lu Y, Dai L et al (2020) Circular RNA circCORO1C promotes laryngeal squamous cell carcinoma progression by modulating the let-7c-5p/PBX3 axis. Mol Cancer 19(1):99. https://doi.org/10.1186/s12943-020-01215-4
Liao L, Chen W, Lai H, Yi X, Wang D (2020) Prognostic nomogram based on immune scores for laryngeal squamous cell cancer. Eur Arch Otorhinolaryngol 278(1):141–148. https://doi.org/10.1007/s00405-020-06189-z
Song L, Zhang S, Yu S, Ma F, Wang B, Zhang C et al (2020) Cellular heterogeneity landscape in laryngeal squamous cell carcinoma. Int J Cancer 147(10):2879–2890. https://doi.org/10.1002/ijc.33192
Li W, Zhao K, Wang Z (2020) Prognostic nomograms based on immune scores for head-neck squamous cell carcinoma patients. Eur Arch Otorhinolaryngol. https://doi.org/10.1007/s00405-020-06358-0
Park G, Kim J, Roh J, Choi S, Nam S, Kim S (2013) Prognostic value of metabolic tumor volume measured by 18F-FDG PET/CT in advanced-stage squamous cell carcinoma of the larynx and hypopharynx. Ann Oncol 24(1):208–214. https://doi.org/10.1093/annonc/mds247
Cui J, Wang L, Zhong W, Chen Z, Tan X, Yang H et al (2020) Development and validation of nomogram to predict risk of survival in patients with laryngeal squamous cell carcinoma. Biosci Rep 40(8):228. https://doi.org/10.1042/bsr20200228
Wang H, An P, Xie E, Wu Q, Fang X, Gao H et al (2017) Characterization of ferroptosis in murine models of hemochromatosis. Hepatology (Baltimore, MD) 66(2):449–465. https://doi.org/10.1002/hep.29117
Yu J, Wang J (2020) Research mechanisms of and pharmaceutical treatments for ferroptosis in liver diseases. Biochimie 180:149–157. https://doi.org/10.1016/j.biochi.2020.11.002
Tang B, Zhu J, Li J, Fan K, Gao Y, Cheng S et al (2020) The ferroptosis and iron-metabolism signature robustly predicts clinical diagnosis, prognosis and immune microenvironment for hepatocellular carcinoma. Cell Commun Signal 18(1):174. https://doi.org/10.1186/s12964-020-00663-1
Liang J, Wang D, Lin H, Chen X, Yang H, Zheng Y et al (2020) A novel Ferroptosis-related gene signature for overall survival prediction in patients with hepatocellular carcinoma. Int J Biol Sci 16(13):2430–2441. https://doi.org/10.7150/ijbs.45050
Yee P, Wei Y, Kim S, Lu T, Chih S, Lawson C et al (2020) Neutrophil-induced ferroptosis promotes tumor necrosis in glioblastoma progression. Nat Commun 11(1):5424. https://doi.org/10.1038/s41467-020-19193-y
Liu H, Hu H, Li G, Zhang Y, Wu F, Liu X et al (2020) Ferroptosis-related gene signature predicts Glioma cell death and Glioma patient progression. Front Cell Dev Biol 8:538. https://doi.org/10.3389/fcell.2020.00538
Lee J, You J, Shin D, Roh J (2020) Inhibition of Glutaredoxin 5 predisposes Cisplatin-resistant head and neck cancer cells to Ferroptosis. Theranostics 10(17):7775–7786. https://doi.org/10.7150/thno.46903
Shin D, Lee J, You J, Kim D, Roh J (2020) Dihydrolipoamide dehydrogenase regulates cystine deprivation-induced ferroptosis in head and neck cancer. Redox Biol 30:101418. https://doi.org/10.1016/j.redox.2019.101418
Hassannia B, Vandenabeele P, Vanden BT (2019) Targeting Ferroptosis to Iron out cancer. Cancer Cell 35(6):830–849. https://doi.org/10.1016/j.ccell.2019.04.002
Lin R, Zhang Z, Chen L, Zhou Y, Zou P, Feng C et al (2016) Dihydroartemisinin (DHA) induces ferroptosis and causes cell cycle arrest in head and neck carcinoma cells. Cancer Lett 381(1):165–175. https://doi.org/10.1016/j.canlet.2016.07.033
Kim EH, Shin D, Lee J, Jung AR, Roh JL (2018) CISD2 inhibition overcomes resistance to sulfasalazine-induced ferroptotic cell death in head and neck cancer. Cancer Lett 432:180–190. https://doi.org/10.1016/j.canlet.2018.06.018
Shen Y, Li X, Zhao B, Xue Y, Wang S, Chen X et al (2018) Iron metabolism gene expression and prognostic features of hepatocellular carcinoma. J Cell Biochem 119(11):9178–9204. https://doi.org/10.1002/jcb.27184
Huang H, Qiu Y, Huang G, Zhou X, Zhou X, Luo W (2019) Value of Ferritin Heavy Chain (FTH1) expression in diagnosis and prognosis of renal cell carcinoma. Med Sci Monit 25:3700–3715. https://doi.org/10.12659/msm.914162
Hu Z, Wang L, Han Y, Li F, Zheng A, Xu Y et al (2019) Ferritin: a potential serum marker for lymph node metastasis in head and neck squamous cell carcinoma. Oncol Lett 17(1):314–322. https://doi.org/10.3892/ol.2018.9642
Brushia RJ, Walsh DA (1999) Phosphorylase kinase: the complexity of its regulation is reflected in the complexity of its structure. Front Biosci 4:D618–D641. https://doi.org/10.2741/brushia
Yang WS, Kim KJ, Gaschler MM, Patel M, Shchepinov MS, Stockwell BR (2016) Peroxidation of polyunsaturated fatty acids by lipoxygenases drives ferroptosis. Proc Natl Acad Sci U S A 113(34):E4966–E4975. https://doi.org/10.1073/pnas.1603244113
Jiang L, Kon N, Li T, Wang SJ, Su T, Hibshoosh H et al (2015) Ferroptosis as a p53-mediated activity during tumour suppression. Nature 520(7545):57–62. https://doi.org/10.1038/nature14344
Jennis M, Kung CP, Basu S, Budina-Kolomets A, Leu JI, Khaku S et al (2016) An African-specific polymorphism in the TP53 gene impairs p53 tumor suppressor function in a mouse model. Genes Dev 30(8):918–930. https://doi.org/10.1101/gad.275891.115
Ye J, Wang Z, Chen X, Jiang X, Dong Z, Hu S et al (2020) YTHDF1-enhanced iron metabolism depends on TFRC m(6)A methylation. Theranostics 10(26):12072–12089. https://doi.org/10.7150/thno.51231
Acknowledgements
The authors thank the efforts of the National Cancer Institute in the creation of the TCGA and GEO databases.
Author information
Authors and Affiliations
Contributions
FH and WF conceived and designed the study; FH and WF collected the data. WF and TC analyzed the data. ZW and DW contributed to the writing and revision of the manuscript. YY and JL participate in external data collection and verification.
Corresponding author
Ethics declarations
Conflict of interest
No potential conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
405_2021_6789_MOESM1_ESM.rar
Supplementary file1 Fig. S1 a, b Prognostic value detection of the gene signature and clinical features via univariate (green) and multivariate (red) Cox regression analysis in TCGA cohort. c, d Prognostic value detection of the gene signature and clinical features via univariate (green) and multivariate (red) Cox regression analysis in GEO cohort. The results identified that our signature risk score was an independent prognostic factor in training and validation cohorts. Fig. S2 a Ferroptosis-related gene signature in TCGA cohort. b, c. The distribution and survival status of patients with risk score in the laryngeal cancer in TCGA cohort. d Ferroptosis-related gene signature in GEO cohort. e, f. The distribution and survival status of patients with risk score in the laryngeal cancer in GEO cohort. (RAR 1231 KB)
Rights and permissions
About this article
Cite this article
Han, F., Li, W., Chen, T. et al. Ferroptosis-related genes for predicting prognosis of patients with laryngeal squamous cell carcinoma. Eur Arch Otorhinolaryngol 278, 2919–2925 (2021). https://doi.org/10.1007/s00405-021-06789-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00405-021-06789-3