Abstract
Purpose
Impaired brain cortices contribute significantly to the pathophysiological mechanisms of post-traumatic olfactory dysfunction (PTOD). This study aimed to use 18F-fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG PET/CT) to measure cerebral cortices' metabolism activity and then to explore their associations with olfaction in patients with PTOD.
Methods
Ethics committee-approved prospective studies included 15 patients with post-traumatic anosmia and 11 healthy volunteers. Olfactory function was assessed using the Sniffin’ Sticks. Participants underwent 18F-FDG PET/CT scan and the image data were collected for the voxel-based whole brain analysis. Furthermore, the standardized uptake value ratio (SUVR) of the whole brain regions was measured and correlated with olfactory function.
Results
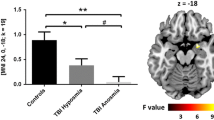
Patients with post-traumatic anosmia showed significantly reduced glucose metabolism in bilateral rectus, bilateral superior and medial orbitofrontal cortex (OFC), bilateral thalamus, left hippocampus and parahippocampus and left superior temporal pole (all p < 0.001). In contrast, patients with post-traumatic anosmia had significantly increased glucose metabolism in the bilateral insula (all p < 0.001). SUVR values among a total of 17 cerebral cortices including frontal, limbic, and temporal regions were significantly and positively correlated with olfactory function. The cerebral cortices with the top three correlations were the right middle frontal OFC (r = 0.765, p = 0.001), right caudate (r = 0.652, p = 0.010) and right putamen (r = 0.623, p = 0.002).
Conclusion
Patients with post-traumatic anosmia presented with distinct patterns of brain metabolism and key cortices that highly associated with the retained olfactory function were identified. The preliminary results further support the potential use of PET imaging for precisely assessing brain metabolism in patients with PTOD.


Similar content being viewed by others
References
Fan LY, Kuo CL, Lirng JF, Shu CH (2015) Investigation of prognostic factors for post-traumatic olfactory dysfunction. J Chin Med Assoc 78:299–303
Hummel T, Whitcroft KL, Andrews P et al (2016) Position paper on olfactory dysfunction. Rhinology 56:1–30
Ahmedy F, Mazlan M, Danaee M, Abu Bakar MZ (2020) Post-traumatic brain injury olfactory dysfunction: factors influencing quality of life. Eur Arch Otorhinolaryngol 277:1343–1351
Bratt M, Skandsen T, Hummel T et al (2018) Frequency and prognostic factors of olfactory dysfunction after traumatic brain injury. Brain Inj 32:1021–1027
Schofield PW, Doty RL (2019) The influence of head injury on olfactory and gustatory function. Handb Clin Neurol 164:409–429
Ogawa T, Rutka J (1999) Olfactory dysfunction in head injured workers. Acta Otolaryngol Suppl 540:50–57
Levin HS, High WM, Eisenberg HM (1985) Impairment of olfactory recognition after closed head injury. Brain 108(Pt 3):579–591
Lötsch J, Reither N, Bogdanov V et al (2015) A brain-lesion pattern based algorithm for the diagnosis of posttraumatic olfactory loss. Rhinology 53:365–370
Whitcroft KL, Hummel T (2019) Clinical diagnosis and current management strategies for olfactory dysfunction: a review. JAMA Otolaryngol Head Neck Surg. 145(9):846–53
Han P, Winkler N, Hummel C, Hähner A, Gerber J, Hummel T (2018) Alterations of brain gray matter density and olfactory bulb volume in patients with olfactory loss after traumatic brain injury. J Neurotrauma 35:2632–2640
Moon WJ, Park M, Hwang M, Kim JK (2018) Functional MRI as an objective measure of olfaction deficit in patients with traumatic anosmia. AJNR Am J Neuroradiol 39:2320–2325
Miao X, Yang L, Gu H et al (2015) Evaluation of post-traumatic anosmia with MRI and chemosensory ERPs. Eur Arch Otorhinolaryngol 272:1945–1953
Han P, Zang Y, Akshita J, Hummel T (2019) Magnetic resonance imaging of human olfactory dysfunction. Brain Topogr 32:987–997
Rice L, Bisdas S (2017) The diagnostic value of FDG and amyloid PET in Alzheimer’s disease—a systematic review. Eur J Radiol 94:16–24
Meyer JH, Cervenka S, Kim MJ, Kreisl WC, Henter ID, Innis RB (2020) Neuroinflammation in psychiatric disorders: PET imaging and promising new targets. Lancet Psychiatry 7:1064–1074
Chiaravalloti A, Pagani M, Micarelli A et al (2015) Cortical activity during olfactory stimulation in multiple chemical sensitivity: a (18)F-FDG PET/CT study. Eur J Nucl Med Mol Imaging 42:733–740
Shin SS, Bales JW, Edward Dixon C, Hwang M (2017) Structural imaging of mild traumatic brain injury may not be enough: overview of functional and metabolic imaging of mild traumatic brain injury. Brain Imaging Behav 11:591–610
Lötsch J, Ultsch A, Eckhardt M, Huart C, Rombaux P, Hummel T (2016) Brain lesion-pattern analysis in patients with olfactory dysfunctions following head trauma. Neuroimage Clin 11:99–105
Yang L, Wei Y, Yu D, Zhang J, Liu Y (2010) Olfactory and gustatory function in healthy adult Chinese subjects. Otolaryngol Head Neck Surg 143:554–560
Tzourio-Mazoyer N, Landeau B, Papathanassiou D et al (2002) Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage 15:273–289
Woo CW, Krishnan A, Wager TD (2014) Cluster-extent based thresholding in fMRI analyses: pitfalls and recommendations. Neuroimage 91:412–419
Lieberman MD, Cunningham WA (2009) Type I and Type II error concerns in fMRI research: re-balancing the scale. Soc Cogn Affect Neurosci 4:423–428
Fjaeldstad A, Fernandes HM, Van Hartevelt TJ et al (2017) Brain fingerprints of olfaction: a novel structural method for assessing olfactory cortical networks in health and disease. Sci Rep 7:42534
Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH (2003) An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage 19:1233–1239
Oh YS, Kim JS, Hwang EJ, Lyoo CH (2018) Striatal dopamine uptake and olfactory dysfunction in patients with early Parkinson’s disease. Parkinsonism Relat Disord 56:47–51
Iannilli E, Gerber J, Frasnelli J, Hummel T (2007) Intranasal trigeminal function in subjects with and without an intact sense of smell. Brain Res 1139:235–244
Joshi A, Han P, Faria V, Larsson M, Hummel T (2020) Neural processing of olfactory-related words in subjects with congenital and acquired olfactory dysfunction. Sci Rep 10:14377
Seubert J, Ohla K, Yokomukai Y, Kellermann T, Lundström JN (2015) Superadditive opercular activation to food flavor is mediated by enhanced temporal and limbic coupling. Hum Brain Mapp 36:1662–1676
Frasnelli J, Lundström JN, Boyle JA, Djordjevic J, Zatorre RJ, Jones-Gotman M (2010) Neuroanatomical correlates of olfactory performance. Exp Brain Res 201:1–11
Rolls ET (2016) Functions of the anterior insula in taste, autonomic, and related functions. Brain Cogn 110:4–19
Frasnelli J, Schuster B, Hummel T (2007) Interactions between olfaction and the trigeminal system: what can be learned from olfactory loss. Cereb Cortex 17:2268–2275
Varney NR, Pinkston JB, Wu JC (2001) Quantitative PET findings in patients with posttraumatic anosmia. J Head Trauma Rehabil 16:253–259
Caminiti F, Ciurleo R, Bramanti P, Marino S (2013) Persistent anosmia in a traumatic brain injury patient: role of orbitofrontal cortex. Brain Inj 27:1715–1718
Courtiol E, Wilson DA (2015) The olfactory thalamus: unanswered questions about the role of the mediodorsal thalamic nucleus in olfaction. Front Neural Circuits 9:49
Kringelbach ML (2005) The human orbitofrontal cortex: linking reward to hedonic experience. Nat Rev Neurosci 6:691–702
Kubota S, Masaoka Y, Sugiyama H et al (2020) Hippocampus and parahippocampus volume reduction associated with impaired olfactory abilities in subjects without evidence of cognitive decline. Front Hum Neurosci 14:556519
Hummel T, Fliessbach K, Abele M et al (2010) Olfactory FMRI in patients with Parkinson’s disease. Front Integr Neurosci 4:125
Lian TH, Zhu WL, Li SW et al (2019) Clinical, structural, and neuropathological features of olfactory dysfunction in patients with Alzheimer’s Disease. J Alzheimers Dis 70:413–423
Brand G, Millot JL, Henquell D (2001) Complexity of olfactory lateralization processes revealed by functional imaging: a review. Neurosci Biobehav Rev 25:159–166
Zelano C, Bensafi M, Porter J et al (2005) Attentional modulation in human primary olfactory cortex. Nat Neurosci 8:114–120
Soudry Y, Lemogne C, Malinvaud D, Consoli SM, Bonfils P (2011) Olfactory system and emotion: common substrates. Eur Ann Otorhinolaryngol Head Neck Dis 128:18–23
Zhou G, Lane G, Cooper SL, Kahnt T, Zelano C (2019) Characterizing functional pathways of the human olfactory system. Elife 8:e47177
Korshunov KS, Blakemore LJ, Trombley PQ (2020) Illuminating and sniffing out the neuromodulatory roles of dopamine in the retina and olfactory bulb. Front Cell Neurosci 14:275
Meunier D, Fonlupt P, Saive AL, Plailly J, Ravel N, Royet JP (2014) Modular structure of functional networks in olfactory memory. Neuroimage 95:264–275
Löhle M, Wolz M, Beuthien-Baumann B et al (2020) Olfactory dysfunction correlates with putaminal dopamine turnover in early de novo Parkinson’s disease. J Neural Transm (Vienna) 127:9–16
Acknowledgments
We thank Junqi Li, Ziwei Zhu and other technicians from the Nuclear Medicine Department of Beijing Anzhen Hospital, for their help and support during the experimental scanning.
Funding
This study was supported by Beijing Hospitals Authority Youth Program (QML20190617), Beijing Science and Technology Nova Program (Z201100006820086), Beijing Hospitals Authority Clinical Medicine Development of Special Funding Support (XMLX202136), Natural Science Foundation of China (82000954), Beijing Hospitals Authority' Mission Plan (SML20190601), and Beijing Scholars Program (No. 051).
Author information
Authors and Affiliations
Contributions
XG drafted the manuscript and analyzed the date. DW revised the manuscript, designed the study and analyzed the date. XL contributed to the methodology. BS analyzed the date. ZS and BN contributed to the methodology and interpretation of data. XZ contributed to the vital instruments and medicines. YW designed the study, revised the manuscript, supervised the study, and obtained the funding.
Corresponding author
Ethics declarations
Conflict of interest
None of the authors has a potential conflict of interest.
Ethics approval
This study was approved by the Ethics Committee at Beijing Anzhen Hospital (Beijing, China; Approval No.2019015X). The study design complied with the criteria of the Declaration of Helsinki for Medical Research involving Human Subjects.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Consent to publish
The authors affirm that human research participants provided informed consent for publication of the images in figures.
Software
MATLAB 2013a, Medical image Analysis, RRID:SCR_013499.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gao, X., Wu, D., Li, X. et al. Altered glucose metabolism of the olfactory-related cortices in anosmia patients with traumatic brain injury. Eur Arch Otorhinolaryngol 278, 4813–4821 (2021). https://doi.org/10.1007/s00405-021-06754-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00405-021-06754-0