Abstract
Purpose
The standard induction chemotherapy for head and neck cancer is TPF [cisplatin (CDDP), docetaxel (DOC), and 5-fluorouracil (5-FU)]. We assessed whether one course of TPF could predict the efficacy of chemoradiotherapy for human papilloma virus (HPV)-related oropharyngeal squamous cell carcinoma.
Methods
We retrospectively reviewed 51 patients with stage III–IV HPV-related oropharyngeal squamous cell carcinoma who received one course of TPF with CDDP 60 mg/m2, DOC 60 mg/m2, and 5-FU 600 mg/m2. We recommended chemoradiotherapy for patients with complete or partial response (CR/PR), and surgery for those with stable or progressive disease (SD/PD). The endpoints were TPF-related adverse events and efficacy, chemoradiotherapy efficacy, and 2-year survival.
Results
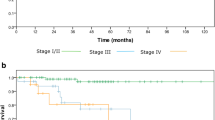
Neutropenia was the most common grade ≥ 3 adverse event (88%). No grade 5 adverse events occurred. TPF achieved CR in 4% of patients (2/51), PR in 73% (37/51), SD in 20% (10/51), and PD in 4% (2/51). Concurrent cetuximab and radiotherapy (bio-radiotherapy, BRT) were administered to 61% of patients (31/51), concurrent CDDP and radiotherapy (CDDP-RT) to 16% (8/51), RT alone to 2% (1/51), and surgery was performed for 22% (11/51). CR was achieved in 85% of the chemoradiotherapy group, and the rate tended to increase with TPF efficacy. CR was achieved in 84% (26/31) of patients receiving BRT, 88% (7/8) receiving CDDP-RT, and 100% (1/1) receiving RT. The 2-year survival rates were 92% overall, and 97% and 79% in the chemoradiotherapy and surgery groups, respectively.
Conclusions
When facing difficulty in deciding between chemoradiotherapy and surgery, one course of TPF may be an effective option.





Similar content being viewed by others
Data availability
Not applicable.
Code availability
Not applicable.
References
Pignon JP, Bourhis J, Domenge C, Designé L (2000) Chemotherapy added to locoregional treatment for head and neck squamous-cell carcinoma: three meta-analyses of updated individual data. MACH-NC Collaborative Group. Meta-analysis of chemotherapy on head and neck cancer. Lancet 355:949–955
Pignon JP, le Maitre A, Maillard E, Bourhis J, MACH-NC Collaborative Group (2009) Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): an update on 93 randomised trials and 17,346 patients. Radiother Oncol 92:4–14
Vermorken JB, Remenar E, van Herpen C, Gorlia T, Mesia R, Degardin M, Stewart JS, Jelic S, Betka J, Preiss JH, van den Wengaert D, Awada A, Cupissol D, Kienzer HR, Rey A, Desaunois I, Bernier J, Lefebvre JL, EORTC 24791/TAX 323 Study Group (2007) Cisplatin, fluorouracil, and docetaxel in unresectable head and neck cancer. N Engl J Med 357:1695–1704
Posner MR, Hershock DM, Blajman CR, Mickiewicz E, Winquist E, Gorbounova V, Tjulandin S, Shin DM, Cullen K, Ervin TJ, Murphy BA, Raez LE, Cohen RB, Spaulding M, Tishler RB, Roth B, Viroglio Rdel C, Venkatesan V, Romanov I, Agarwala S, Harter KW, Dugan M, Cmelak A, Markoe AM, Read PW, Steinbrenner L, Colevas AD, Norris CM Jr, Haddad RI, TAX 324 Study Group (2007) Cisplatin and fluorouracil alone or with docetaxel in head and neck cancer. N Engl J Med 357:1705–1715
Ghi MG, Paccagnella A, Ferrari D, Foa P, Alterio D, Codecà C, Nolè F, Verri E, Orecchia R, Morelli F, Parisi S, Mastromauro C, Mione CA, Rossetto C, Polsinelli M, Koussis H, Loreggian L, Bonetti A, Campostrini F, Azzarello G, D’Ambrosio C, Bertoni F, Casanova C, Emiliani E, Guaraldi M, Bunkheila F, Bidoli P, Niespolo RM, Gava A, Massa E, Frattegiani A, Valduga F, Pieri G, Cipani T, Da Corte D, Chiappa F, Rulli E, GSTTC (Gruppo di Studio Tumori della Testa e del Collo) Italian Study Group (2007) Induction TPF followed by concomitant treatment versus concomitant treatment alone in locally advanced head and neck cancer. A phase II-III trial. Ann Oncol 28:2206–2212
Haddad R, O’Neill A, Rabinowits G, Tishler R, Khuri F, Adkins D, Clark J, Sarlis N, Lorch J, Beitler J, Limaye S, Riley S, Posner M (2013) Induction chemotherapy followed by concurrent chemoradiotherapy (sequential chemoradiotherapy) versus concurrent chemoradiotherapy alone in locally advanced head and neck cancer (PARADIGM): a randomised phase 3 trial. Lancet Oncol 14:257–264
Cohen EE, Karrison TG, Kocherginsky M, Mueller J, Egan R, Huang CH, Brockstein BE, Agulnik MB, Mittal BB, Yunus F, Samant S, Raez LE, Mehra R, Kumar P, Ondrey F, Marchand P, Braegas B, Seiwert TY, Villaflor VM, Haraf DJ, Vokes EE (2014) Phase III randomized trial of induction chemotherapy in patients with N2 or N3 locally advanced head and neck cancer. J Clin Oncol 32:2735–2743
Hitt R, Grau JJ, Lopez-Pousa A, Berrocal A, García-Girón C, Irigoyen A, Sastre J, Martínez-Trufero J, Brandariz Castelo JA, Verger E, Cruz-Hernández JJ, Spanish Head and Neck Cancer Cooperative Group (TTCC) (2014) A randomized phase III trial comparing induction chemotherapy followed by chemoradiotherapy versus chemoradiotherapy alone as treatment of unresectable head and neck cancer. Ann Oncol 25:216–225
Kiong KL, de Souza NN, Sultana R, Iyer NG (2018) Meta-analysis of induction chemotherapy as a selection marker for chemoradiation in the head and neck. Laryngoscope 128:1594–1601
Urba S, Wolf G, Eisbruch A, Worden F, Lee J, Bradford C, Teknos T, Chepeha D, Prince M, Hogikyan N, Taylor J (2006) Single-cycle induction chemotherapy selects patients with advanced laryngeal cancer for combined chemoradiation: a new treatment paradigm. J Clin Oncol 24:593–598
Vainshtein JM, Wu VF, Spector ME, Bradford CR, Wolf GT, Worden FP (2013) Chemoselection: a paradigm for optimization of organ preservation in locally advanced larynx cancer. Expert Rev Anticancer Ther 13:1053–1064
Worden FP, Kumar B, Lee JS, Wolf GT, Cordell KG, Taylor JMG, Urba SG, Eisbruch A, Teknos TN, Chepeha DB, Prince ME, Tsien CI, D’Silva NJ, Yang K, Kurnit DM, Mason HL, Miller TH, Wallace NE, Bradford CR, Carey TE (2008) Chemoselection as a strategy for organ preservation in advanced oropharynx cancer: response and survival positively associated with HPV16 copy number. J Clin Oncol 26:3138–3146
Edge SB, Byrd DR, Compton CC, Fritz AG, Greene F, Trotti A (eds) (2010) AJCC cancer staging manual, 7th edn. Springer, New York
Japan Society for Head and Neck Cancer (2018) General rules for clinical studies on head and neck cancer, 6th edn. Kanehara-shuppan, Tokyo
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J (2009) New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer 45:228–247
Bonner JA, Harari PM, Giralt J, Azarnia N, Shin DM, Cohen RB, Jones CU, Sur R, Raben D, Jassem J, Ove R, Kies MS, Baselga J, Youssoufian H, Amellal N, Rowinsky EK, Ang KK (2006) Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med 354:567–578
Hirasawa K, Okamoto I, Motohashi R, Sato H, Takase S, Agata A, Takeda A, Tsukahara K (2017) The efficiency and adverse events of radiotherapy with cetuximab for Japanese head and neck cancer patients. Auris Nasus Larynx 44:724–728
Amin MB, Edge SB, Greene FL, Byrd DR, Brookland RK, Washington MK, Gershenwald JE, Compton CC, Hess KR, Sullivan DC, Jessup JM, Brierley JD, Gaspar LE, Schilsky RL, Balch CM, Winchester DP, Asare EA, Madera M, Gress DM, Meyer LR (eds) (2017) AJCC cancer staging manual, 8th edn. Springer, New York
Ang KK, Harris J, Wheeler R, Weber R, Rosenthal DI, Nguyen-Tân PF, Westra WH, Chung CH, Jordan RC, Lu C, Kim H, Axelrod R, Silverman CC, Redmond KP, Gillison ML (2010) Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med 363:24–35
El-Naggar AK, Westra WH (2012) p16 expression as a surrogate marker for HPV-related oropharyngeal carcinoma: a guide for interpretative relevance and consistency. Head Neck 34:459–461
Gillison ML, Trotti AM, Harris J, Eisbruch A, Harari PM, Adelstein DJ, Jordan RCK, Zhao W, Sturgis EM, Burtness B, Ridge JA, Ringash J, Galvin J, Yao M, Koyfman SA, Blakaj DM, Razaq MA, Colevas AD, Beitler JJ, Jones CU, Dunlap NE, Seaward SA, Spencer S, Galloway TJ, Phan J, Dignam JJ, Le QT (2019) Radiotherapy plus cetuximab or cisplatin in human papillomavirus-positive oropharyngeal cancer (NRG Oncology RTOG 1016): a randomised, multicentre, non-inferiority trial. Lancet 393:40–50
Mehanna H, Robinson M, Hartley A, Kong A, Foran B, Fulton-Lieuw T, Dalby M, Mistry P, Sen M, O’Toole L, Al Booz H, Dyker K, Moleron R, Whitaker S, Brennan S, Cook A, Griffin M, Aynsley E, Rolles M, De Winton E, Chan A, Srinivasan D, Nixon I, Grumett J, Leemans CR, Buter J, Henderson J, Harrington K, McConkey C, Gray A, Dunn J, De-ESCALaTE HPV Trial Group (2019) Radiotherapy plus cisplatin or cetuximab in low-risk human papillomavirus-positive oropharyngeal cancer (De-ESCALaTE HPV): an open-label randomised controlled phase 3 trial. Lancet 393:51–60
Saito Y, Ando M, Omura G, Yasuhara K, Yoshida M, Takahashi W, Yamasoba T (2016) Induction chemotherapy for p16 positive oropharyngeal squamous cell carcinoma. Laryngoscope Investig Otolaryngol 1:28–32
Misiukiewicz K, Gupta V, Miles BA, Bakst R, Genden E, Selkridge I, Surgeon JT, Rainey H, Camille N, Roy E, Zhang D, Ye F, Jia R, Moshier E, Bonomi M, Hwang M, Som P, Posner MR (2019) Standard of care vs reduced-dose chemoradiation after induction chemotherapy in HPV+ oropharyngeal carcinoma patients: The Quarterback trial. Oral Oncol 95:170–177
Chidambaram S, Nakken ER, Kennedy W, Thorstad WL, Chen SY, Pipkorn P, Zevallos JP, Mazul AL (2020) Prognostic significance of smoking in human papillomavirus-positive oropharyngeal cancer under American joint committee on eighth edition cancer stage. Laryngoscope 130:1961–1966
Nakano K, Sato Y, Toshiyasu T, Sato Y, Inagaki L, Tomomatsu J, Sasaki T, Shimbashi W, Fukushima H, Yonekawa H, Mitani H, Kawabata K, Takahashi S (2016) Predictive factors of head and neck squamous cell carcinoma patient tolerance to high-dose cisplatin in concurrent chemoradiotherapy. Mol Clin Oncol 4:303–309
Funding
The authors have no financial relationships to disclose.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to disclose.
Ethical approval
The study was approved by the ethics committee of Tokyo Medical University (approval number T2020-0074). We conducted this study in compliance with the Declaration of Helsinki.
Consent to participate
We obtained the written consent of all participating patients.
Consent for publication
Patients signed informed consent regarding publishing their data.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kondo, T., Tsukahara, K., Okamoto, I. et al. Predicting the efficacy of chemoradiotherapy for locally advanced human papilloma virus-related oropharyngeal squamous cell carcinoma using one course of TPF chemotherapy. Eur Arch Otorhinolaryngol 278, 3497–3506 (2021). https://doi.org/10.1007/s00405-020-06549-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00405-020-06549-9