Abstract
Purpose
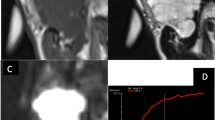
To assess the added value of susceptibility-weighted imaging (SWI) to diffusion-weighted imaging (DWI) in the characterization of parotid gland tumors.
Methods
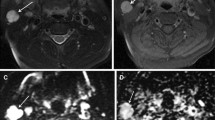
Seventy-eight patients with pathologically confirmed parotid gland tumors, who underwent DWI and SWI for pre-surgery evaluation, were enrolled. Apparent diffusion coefficient (ADC) and degree of intratumoral susceptibility signal intensity (ITSS) were measured and compared between benign and malignant groups, and among pleomorphic adenoma (PA), Warthin tumor (WT) and malignant tumor (MT). Independent sample t test, one-way analysis of variance and receiver operating characteristic curve analysis were used for statistical analyses.
Results
Benign parotid gland tumor showed a significantly higher mean ADC value than malignant tumors (0.836 ± 0.350 vs 0.592 ± 0.163, p = 0.001). Setting an average ADC value of 0.679 as the cut-off value, optimal differentiating performance could be obtained (AUC, 0.700; sensitivity, 62.69%; specificity, 81.82%) for differentiating malignant from benign tumors. PA showed significantly higher mean ADC and less ITSS than WT (ADC, p < 0.001; ITSS, p = 0.033) and MT (ADC, p < 0.001; ITSS, p = 0.024), while the difference between WT and MT was not significant (ADC, p = 0.826; ITSS, p = 0.539). After integration with ITSS, the diagnostic performance of ADC was improved for differentiating PA from WT (AUC 0.921 vs 0.873) and from MT (AUC 0.906 vs 0.882).
Conclusion
SWI could provide added information to DWI and serve as a supplementary imaging marker for the characterization of parotid gland tumors.



Similar content being viewed by others
References
Erkan Gökçe (2020) Multiparametric magnetic resonance imaging for the diagnosis and differential diagnosis of parotid gland tumors. J Magn Reson Imaging (Online ahead of print)
Qian W, Xu XQ, Zhu LN et al (2019) Preliminary study of using diffusion kurtosis imaging for characterizing parotid gland tumors. Acta Radiol 60:887–894
Yamamoto T, Kimura H, Hayashi K, Imamura Y, Mori M (2018) Pseudo-continuous arterial spin labeling MR images in Warthin tumors and pleomorphic adenomas of the parotid gland: qualitative and quantitative analyses and their correlation with histopathologic and DWI and dynamic contrast enhanced MRI findings. Neuroradiology 60:803–812
Elmokadem AH, Abdel Khalek AM, Abdel Wahab RM et al (2019) Diagnostic accuracy of multiparametric magnetic resonance imaging for differentiation between parotid neoplasms. Can Assoc Radiol J 70:264–272
Tao X, Yang G, Wang P et al (2017) The value of combining conventional, diffusion-weighted and dynamic contrast-enhanced MR imaging for the diagnosis of parotid gland tumours. Dentomaxillofac Radiol 46:20160434
Christe A, Waldherr C, Hallett R, Zbaeren P, Thoeny H (2011) MR imaging of parotid tumors: typical lesion characteristics in MR imaging improve discrimination between benign and malignant disease. AJNR Am J Neuroradiol 32:1202–1207
Wu Q, Zhu LN, Jiang JS, Bu SS, Xu XQ, Wu FY (2019) Characterization of parotid gland tumors using T2 mapping imaging: initial findings. Acta Radiol (Online ahead of print)
Zhu L, Wang J, Shi H, Tao X (2019) Multimodality fMRI with perfusion, diffusion-weighted MRI and 1H-MRS in the diagnosis of lympho-associated benign and malignant lesions of the parotid gland. J Magn Reson Imaging 49:423–432
Xu Z, Zheng S, Pan A, Cheng X, Gao M (2019) A multiparametric analysis based on DCE-MRI to improve the accuracy of parotid tumor discrimination. Eur J Nucl Med Mol Imaging 46:2228–2234
Ma G, Zhu LN, Su GY et al (2018) Histogram analysis of apparent diffusion coefficient maps for differentiating malignant from benign parotid gland tumors. Eur Arch Otorhinolaryngol 275:2151–2157
Kato H, Kanematsu M, Watanabe H et al (2015) Perfusion imaging of parotid gland tumors: usefulness of arterial spin labeling for differentiating Warthin's tumors. Eur Radiol 25:3247–3254
Böker SM, Adams LC, Bender YY et al (2019) Differentiation of predominantly osteoblastic and osteolytic spine metastases by using susceptibility-weighted MRI. Radiology 290:146–154
Zhang S, Chiang GC, Knapp JM et al (2019) Grading meningiomas utilizing multiparametric MRI with inclusion of susceptibility weighted imaging and quantitative susceptibility mapping. J Neuroradiol (Online ahead of print)
Yang X, Zhu J, Dai Y et al (2019) Multi-parametric effect in predicting tumor histological grade by using susceptibility weighted magnetic resonance imaging in tongue squamous cell carcinoma. BMC Med Imaging 19:24
Su CQ, Lu SS, Han QY, Zhou MD, Hong XN (2019) Intergrating conventional MRI, texture analysis of dynamic contrast-enhanced MRI, and susceptibility weighted imaging for glioma Grading. Acta Radiol 60:777–787
Saini J, Gupta PK, Awasthi A et al (2018) Multiparametric imaging-based differentiation of lymphoma and glioblastoma: using T1-perfusion, diffusion, and susceptibility-weighted MRI. Clin Radiol 73:986.e7–986.e15
Zhang W, Zuo Z, Huang X, Jin G, Su D (2018) Value of diffusion-weighted imaging combined with susceptibility-weighted imaging in differentiating benign from malignant parotid gland lesions. Med Sci Monit 24:4610–4616
Park SM, Kim HS, Jahng G-H, Ryu C-W, Kim SY (2010) Combination of high-resolution susceptibility-weighted imaging and the apparent diffusion coefficient: added value to brain tumour imaging and clinical feasibility of non-contrast MRI at 3 T. Br J Radiol 83:466–475
DeLong ER, DeLong DM, Clarke-Pearson DL (1988) Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 44:837–845
Xu XQ, Hu H, Liu H et al (2017) Benign and malignant orbital lymphoproliferative disorders: differentiating using multiparametric MRI at 3.0 T. J Magn Reson Imaging 45:167–176
Yuan Y, Tang W, Tao X (2016) Parotid gland lesions: separate and combined diagnostic value of conventional MRI, diffusion-weighted imaging and dynamic contrast-enhanced MRI. Br J Radiol 89:20150912
Matsushima N, Maeda M, Takamura M, Takeda K (2007) Apparent diffusion coefficients of benign and malignant salivary gland tumors. Comparison to histopathological findings. J Neuroradiol 34:183–189
Khalek Abdel Razek AA (2018) Characterization of salivary gland tumours with diffusion tensor imaging. Dentomaxillofac Radiol 47:20170343
King AD, Yeung DK, Ahuja AT et al (2005) Salivary gland tumors at in vivo proton MR spectroscopy. Radiology 237:563–569
Acknowledgements
We want to express our thanks to Yong-Ming Dai from United Imaging Healthcare for his help in MRI protocol setting and manuscript correction.
Funding
This work was supported by National Natural Science Foundation of China (81771796 to FY Wu), and Jiangsu Province’s Young Medical Talents Program (QNRC2016560 to Xu XQ).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This study was approved by the institutional review board of our hospital and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Written informed consent was waived due to the nature of retrospective study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jiang, JS., Zhu, LN., Chen, W. et al. Added value of susceptibility-weighted imaging to diffusion-weighted imaging in the characterization of parotid gland tumors. Eur Arch Otorhinolaryngol 277, 2839–2846 (2020). https://doi.org/10.1007/s00405-020-05985-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00405-020-05985-x