Abstract
Purpose
Thyroid nodules are of common occurrence in the general population. About a fourth of these nodules are indeterminate on aspiration cytology placing many a patient at risk of unwanted surgery. The purpose of this review is to discuss various molecular markers described to date and place their role in proper perspective. This review covers the fundamental role of the signaling pathways and genetic changes involved in thyroid carcinogenesis. The current literature on the prognostic significance of these markers is also described.
Methods
PubMed was used to search relevant articles. The key terms “thyroid nodules”, “thyroid cancer papillary”, “carcinoma papillary follicular”, “carcinoma papillary”, “adenocarcinoma follicular” were searched in MeSH, and “molecular markers”, “molecular testing”, mutation, BRAF, RAS, RET/PTC, PAX 8, miRNA, NIFTP in title and abstract fields. Multiple combinations were done and a group of experts in the subject from the International Head and Neck Scientific Group extracted the relevant articles and formulated the review.
Results
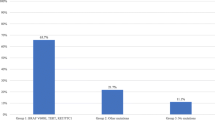
There has been considerable progress in the understanding of thyroid carcinogenesis and the emergence of numerous molecular markers in the recent years with potential to be used in the diagnostic algorithm of these nodules. However, their precise role in routine clinical practice continues to be a contentious issue. Majority of the studies in this context are retrospective and impact of these mutations is not independent of other prognostic factors making the interpretation difficult.
Conclusion
The prevalence of these mutations in thyroid nodule is high and it is a continuously evolving field. Clinicians should stay informed as recommendation on the use of these markers is expected to evolve.
Similar content being viewed by others
References
Ezzat S, Sarti DA, Cain DR, Braunstein GD (1994) Thyroid incidentalomas. Prevalence by palpation and ultrasonography. Arch Intern Med 154(16):1838–1840
Vaccarella S, Franceschi S, Bray F, Wild CP, Plummer M, Dal Maso L (2016) Worldwide thyroid-cancer epidemic? The increasing impact of overdiagnosis. N Engl J Med 375(7):614–617
Haugen BR, Alexander EK, Bible KC et al (2016) 2015 American thyroid association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American thyroid association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid 26(1):1–133
Wang C-CC, Friedman L, Kennedy GC et al (2011) A large multicenter correlation study of thyroid nodule cytopathology and histopathology. Thyroid 21(3):243–251
Nayar R, Ivanovic M (2009) The indeterminate thyroid fine-needle aspiration: experience from an academic center using terminology similar to that proposed in the 2007 National Cancer Institute Thyroid Fine Needle Aspiration State of the Science Conference. Cancer 117(3):195–202
Francis GL, Waguespack SG, Bauer AJ et al (2015) Management guidelines for children with thyroid nodules and differentiated thyroid cancer. Thyroid 25(7):716–759
Cancer Genome Atlas Research N (2014) Integrated genomic characterization of papillary thyroid carcinoma. Cell 159(3):676–690
Cohen Y, Xing M, Mambo E et al (2003) BRAF mutation in papillary thyroid carcinoma. J Natl Cancer Inst 95(8):625–627
Xing M (2013) Molecular pathogenesis and mechanisms of thyroid cancer. Nat Rev Cancer 13(3):184–199
Rabes HM, Demidchik EP, Sidorow JD et al (2000) Pattern of radiation-induced RET and NTRK1 rearrangements in 191 post-chernobyl papillary thyroid carcinomas: biological, phenotypic, and clinical implications. Clin Cancer Res 6(3):1093–1103
Tallini G, Asa SL (2001) RET oncogene activation in papillary thyroid carcinoma. Adv Anat Pathol 8(6):345–354
Mehta V, Nikiforov YE, Ferris RL (2013) Use of molecular biomarkers in FNA specimens to personalize treatment for thyroid surgery. Head Neck 35(10):1499–1506
Vander Poorten V, Hens G, Delaere P (2013) Thyroid cancer in children and adolescents. Curr Opin Otolaryngol Head Neck Surg 21(2):135–142
Thomas GA, Bunnell H, Cook HA et al (1999) High prevalence of RET/PTC rearrangements in Ukrainian and Belarussian post-Chernobyl thyroid papillary carcinomas: a strong correlation between RET/PTC3 and the solid-follicular variant. J Clin Endocrinol Metab 84(11):4232–4238
Adeniran AJ, Zhu Z, Gandhi M et al (2006) Correlation between genetic alterations and microscopic features, clinical manifestations, and prognostic characteristics of thyroid papillary carcinomas. Am J Surg Pathol 30(2):216–222
Croyle M, Akeno N, Knauf JA et al (2008) RET/PTC-induced cell growth is mediated in part by epidermal growth factor receptor (EGFR) activation: evidence for molecular and functional interactions between RET and EGFR. Cancer Res 68(11):4183–4191
Kroll TG, Sarraf P, Pecciarini L et al (2000) PAX8-PPARgamma1 fusion oncogene in human thyroid carcinoma [corrected]. Science 289(5483):1357–1360
Dwight T, Thoppe SR, Foukakis T et al (2003) Involvement of the PAX8/peroxisome proliferator-activated receptor gamma rearrangement in follicular thyroid tumors. J Clin Endocrinol Metab 88(9):4440–4445
Castro P, Rebocho AP, Soares RJ et al (2006) PAX8-PPARgamma rearrangement is frequently detected in the follicular variant of papillary thyroid carcinoma. J Clin Endocrinol Metab 91(1):213–220
Nikiforov YE (2011) Molecular diagnostics of thyroid tumors. Arch Pathol Lab Med 135(5):569–577
Liu X, Bishop J, Shan Y et al (2013) Highly prevalent TERT promoter mutations in aggressive thyroid cancers. Endocr Relat Cancer 20(4):603–610
Acquaviva G, Visani M, Repaci A et al (2018) Molecular pathology of thyroid tumours of follicular cells: a review of genetic alterations and their clinicopathological relevance. Histopathology 72(1):6–31
Li X, Abdel-Mageed AB, Mondal D, Kandil E (2013) MicroRNA expression profiles in differentiated thyroid cancer, a review. Int J Clin Exp Med 6(1):74–80
Pallante P, Visone R, Ferracin M et al (2006) MicroRNA deregulation in human thyroid papillary carcinomas. Endocr Relat Cancer 13(2):497–508
Nikiforova MN, Tseng GC, Steward D, Diorio D, Nikiforov YE (2008) MicroRNA expression profiling of thyroid tumors: biological significance and diagnostic utility. J Clin Endocrinol Metab 93(5):1600–1608
Cibas ES, Ali SZ, Conference NCITFSotS (2009) The Bethesda system for reporting thyroid cytopathology. Am J Clin Pathol 132(5):658–665
Steward DL, Kloos RT (2014) Clinical diagnostic gene expression thyroid testing. Otolaryngol Clin North Am 47(4):573–593
Nikiforov YE, Seethala RR, Tallini G et al (2016) Nomenclature revision for encapsulated follicular variant of papillary thyroid carcinoma: a paradigm shift to reduce overtreatment of indolent tumors. JAMA Onco l2(8):1023–1029
Baloch ZW, Seethala RR, Faquin WC et al (2016) Noninvasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP): a changing paradigm in thyroid surgical pathology and implications for thyroid cytopathology. Cancer Cytopathol 124(9):616–620
Zhao L, Dias-Santagata D, Sadow PM, Faquin WC (2017) Cytological, molecular, and clinical features of noninvasive follicular thyroid neoplasm with papillary-like nuclear features versus invasive forms of follicular variant of papillary thyroid carcinoma. Cancer Cytopathol 125(5):323–331
Maletta F, Massa F, Torregrossa L et al (2016) Cytological features of “noninvasive follicular thyroid neoplasm with papillary-like nuclear features” and their correlation with tumor histology. Hum Pathol 54:134–142
Cibas ES, Ali SZ (2017) The 2017 Bethesda system for reporting thyroid cytopathology. Thyroid 27(11):1341–1346
Nikiforova MN, Nikiforov YE (2009) Molecular diagnostics and predictors in thyroid cancer. Thyroid 19(12):1351–1361
Nikiforov YE (2017) Role of molecular markers in thyroid nodule management: then and now. Endocr Pract 23(8):979–988
Nikiforov YE, Ohori NP, Hodak SP et al (2011) Impact of mutational testing on the diagnosis and management of patients with cytologically indeterminate thyroid nodules: a prospective analysis of 1056 FNA samples. J Clin Endocrinol Metab 96(11):3390–3397
Yip L, Ferris RL (2014) Clinical application of molecular testing of fine-needle aspiration specimens in thyroid nodules. Otolaryngol Clin North Am 47(4):557–571
Esapa CT, Johnson SJ, Kendall-Taylor P, Lennard TW, Harris PE (1999) Prevalence of Ras mutations in thyroid neoplasia. Clin Endocrinol (Oxf) 50(4):529–535
Zhu Z, Gandhi M, Nikiforova MN, Fischer AH, Nikiforov YE (2003) Molecular profile and clinical-pathologic features of the follicular variant of papillary thyroid carcinoma. An unusually high prevalence of ras mutations. Am J Clin Pathol 120(1):71–77
Gupta N, Dasyam AK, Carty SE et al (2013) RAS mutations in thyroid FNA specimens are highly predictive of predominantly low-risk follicular-pattern cancers. J Clin Endocrinol Metab 98(5):E914-22
Paulson VA, Shivdasani P, Angell TE et al (2017) Noninvasive follicular thyroid neoplasm with papillary-like nuclear features accounts for more than half of “carcinomas” harboring RAS mutations. Thyroid 27(4):506–511
Wong KS, Angell TE, Strickland KC et al (2016) Noninvasive follicular variant of papillary thyroid carcinoma and the Afirma gene-expression classifier. Thyroid 26(7):911–915
Zhu Z, Ciampi R, Nikiforova MN, Gandhi M, Nikiforov YE (2006) Prevalence of RET/PTC rearrangements in thyroid papillary carcinomas: effects of the detection methods and genetic heterogeneity. J Clin Endocrinol Metab 91(9):3603–3610
Fenton CL, Lukes Y, Nicholson D, Dinauer CA, Francis GL, Tuttle RM (2000) The ret/PTC mutations are common in sporadic papillary thyroid carcinoma of children and young adults. J Clin Endocrinol Metab 85(3):1170–1175
Cheung CC, Carydis B, Ezzat S, Bedard YC, Asa SL (2001) Analysis of ret/PTC gene rearrangements refines the fine needle aspiration diagnosis of thyroid cancer. J Clin Endocrinol Metab 86(5):2187–2190
Elisei R, Romei C, Vorontsova T et al (2001) RET/PTC rearrangements in thyroid nodules: studies in irradiated and not irradiated, malignant and benign thyroid lesions in children and adults. J Clin Endocrinol Metab 86(7):3211–3216
Alexander EK, Kennedy GC, Baloch ZW et al (2012) Preoperative diagnosis of benign thyroid nodules with indeterminate cytology. N Engl J Med 367(8):705–715
Zhang M, Lin O (2016) Molecular testing of thyroid nodules: a review of current available tests for fine-needle aspiration specimens. Arch Pathol Lab Med 140(12):1338–1344
Giordano TJ, Beaudenon-Huibregtse S, Shinde R et al (2014) Molecular testing for oncogenic gene mutations in thyroid lesions: a case-control validation study in 413 postsurgical specimens. Hum Pathol 45(7):1339–1347
Labourier E, Shifrin A, Busseniers AE et al (2015) Molecular testing for miRNA, mRNA, and DNA on fine-needle aspiration improves the preoperative diagnosis of thyroid nodules with indeterminate cytology. J Clin Endocrinol Metab 100(7):2743–2750
Nikiforova MN, Wald AI, Roy S, Durso MB, Nikiforov YE (2013) Targeted next generation sequencing panel (ThyroSeq) for detection of mutations in thyroid cancer. J Clin Endocrinol Metab 98(11):E1852–E1860
Nikiforov YE, Carty SE, Chiosea SI et al (2014) Highly accurate diagnosis of cancer in thyroid nodules with follicular neoplasm/suspicious for a follicular neoplasm cytology by ThyroSeq v2 next-generation sequencing assay. Cancer 120(23):3627–3634
Nikiforova MN, Mercurio S, Wald AI et al (2018) Analytical performance of the ThyroSeq v3 genomic classifier for cancer diagnosis in thyroid nodules. Cancer. https://doi.org/10.1002/cncr.31245
Benjamin H, Schnitzer-Perlman T, Shtabsky A et al (2016) Analytical validity of a microRNA-based assay for diagnosing indeterminate thyroid FNA smears from routinely prepared cytology slides. Cancer Cytopathol 124(10):711–721
Lithwick-Yanai G, Dromi N, Shtabsky A et al (2017) Multicentre validation of a microRNA-based assay for diagnosing indeterminate thyroid nodules utilising fine needle aspirate smears. J Clin Pathol 70(6):500–507
Lee J-H, Lee E-S, Kim Y-S (2007) Clinicopathologic significance of BRAF V600E mutation in papillary carcinomas of the thyroid: a meta-analysis. Cancer 110(1):38–46
Kim TH, Park YJ, Lim JA et al (2012) The association of the BRAF (V600E) mutation with prognostic factors and poor clinical outcome in papillary thyroid cancer: a meta-analysis. Cancer 118(7):1764–1773
Vuong HG, Duong UNP, Altibi AMAet al (2017) A meta-analysis of prognostic roles of molecular markers in papillary thyroid carcinoma. Endocr Connect 6(3):R8–R17
Kebebew E, Weng J, Bauer J et al (2007) The prevalence and prognostic value of BRAF mutation in thyroid cancer. Ann Surg 246(3):466–470
Song YS, Lim JA, Park YJ (2015) Mutation profile of well-differentiated thyroid cancer in Asians. Endocrinology Metabolism 30(3):252–262
Lupi C, Giannini R, Ugolini C et al (2007) Association of BRAF V600E mutation with poor clinicopathological outcomes in 500 consecutive cases of papillary thyroid carcinoma. J Clin Endocrinol Metab 92(11):4085–4090
Xing M, Westra WH, Tufano RP et al (2005) BRAF mutation predicts a poorer clinical prognosis for papillary thyroid cancer. J Clin Endocrinol Metab 90(12):6373–6379
Howell GM, Nikiforova MN, Carty SE et al (2013) BRAF V600E mutation independently predicts central compartment lymph node metastasis in patients with papillary thyroid cancer. Ann Surg Oncol 20(1):47–52
Xing M, Alzahrani AS, Carson KA et al (2013) Association between BRAF V600E mutation and mortality in patients with papillary thyroid cancer. JAMA 309(14):1493–1501
Park YJ, Kim YA, Lee YJ et al (2010) Papillary microcarcinoma in comparison with larger papillary thyroid carcinoma in BRAF (V600E) mutation, clinicopathological features, and immunohistochemical findings. Head Neck 32(1):38–45
Lee X, Gao M, Ji Y et al (2009) Analysis of differential BRAF(V600E) mutational status in high aggressive papillary thyroid microcarcinoma. Ann Surg Oncol 16(2):240–245
Lin K-L, Wang O-C, Zhang X-H, Dai X-X, Hu X-Q, Qu J-M (2010) The BRAF mutation is predictive of aggressive clinicopathological characteristics in papillary thyroid microcarcinoma. Ann Surg Oncol 17(12):3294–3300
Li F, Chen G, Sheng C et al (2015) BRAFV600E mutation in papillary thyroid microcarcinoma: a meta-analysis. Endocr Relat Cancer 22(2):159–168
Elisei R, Viola D, Torregrossa L et al (2012) The BRAF(V600E) mutation is an independent, poor prognostic factor for the outcome of patients with low-risk intrathyroid papillary thyroid carcinoma: single-institution results from a large cohort study. J Clin Endocrinol Metab 97(12):4390–4398
Xing M, Alzahrani AS, Carson KA et al (2015) Association between BRAF V600E mutation and recurrence of papillary thyroid cancer. J Clin Oncol 33(1):42–50
Elisei R, Ugolini C, Viola D et al (2008) BRAF(V600E) mutation and outcome of patients with papillary thyroid carcinoma: a 15-year median follow-up study. J Clin Endocrinol Metab 93(10):3943–3949
Henke LE, Pfeifer JD, Ma C et al (2015) BRAF mutation is not predictive of long-term outcome in papillary thyroid carcinoma. Cancer Med 4(6):791–799
Tufano RP, Teixeira GV, Bishop J, Carson KA, Xing M (2012) BRAF mutation in papillary thyroid cancer and its value in tailoring initial treatment: a systematic review and meta-analysis. Medicine (Baltimore) 91(5):274–286
Basolo F, Pisaturo F, Pollina LE et al (2000) N-ras mutation in poorly differentiated thyroid carcinomas: correlation with bone metastases and inverse correlation to thyroglobulin expression. Thyroid 10(1):19–23
Garcia-Rostan G, Zhao H, Camp RL et al (2003) ras mutations are associated with aggressive tumor phenotypes and poor prognosis in thyroid cancer. J Clin Oncol 21(17):3226–3235
Mayr B, Brabant G, Goretzki P, Ruschoff J, Dietmaier W, Dralle H (1997) ret/PTC-1, -2, and -3 oncogene rearrangements in human thyroid carcinomas: implications for metastatic potential? J Clin Endocrinol Metab 82(4):1306–1307
Nikiforov YE (2002) RET/PTC rearrangement in thyroid tumors. Endocr Pathol Spring 13(1):3–16
Landa I, Ibrahimpasic T, Boucai L et al (2016) Genomic and transcriptomic hallmarks of poorly differentiated and anaplastic thyroid cancers. J Clin Invest 126(3):1052–1066
Yip L, Farris C, Kabaker AS et al (2012) Cost impact of molecular testing for indeterminate thyroid nodule fine-needle aspiration biopsies. J Clin Endocrinol Metab 97(6):1905–1912
Bernet V, Hupart KH, Parangi S, Woeber KA (2014) AACE/ACE disease state commentary: molecular diagnostic testing of thyroid nodules with indeterminate cytopathology. Endocr Pract 20(4):360–363
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no competing interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
This article was written by members and invitees of the International Head and Neck Scientific Group (http://www.IHNSG.com).
Rights and permissions
About this article
Cite this article
D’Cruz, A.K., Vaish, R., Vaidya, A. et al. Molecular markers in well-differentiated thyroid cancer. Eur Arch Otorhinolaryngol 275, 1375–1384 (2018). https://doi.org/10.1007/s00405-018-4944-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00405-018-4944-1