Abstract
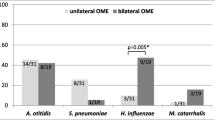
Otitis media with effusion (OME) is a highly prevalent disease in children, but the exact pathogenesis and role of bacteria are still not well understood. This study aimed to investigate the presence of otopathogenic bacteria in the middle ear effusion (MEE) and adenoid of children with chronic OME (COME), and to investigate in vivo whether these bacteria, especially Haemophilus influenzae, are organized as a biofilm in the middle ear fluid. MEE and adenoid samples were collected from 21 patients with COME. Extensive bacterial culturing and genotyping was performed on all middle ear and adenoid samples. Fluorescence in situ hybridization (FISH) and confocal laser scanning microscopy (CLSM) was used to visualize possible biofilm structures for a selection of middle ear effusion samples. 34 MEE samples were collected from 21 patients of which 64.7 % were culture positive for bacteria and 47.0 % were culture positive for Haemophilus influenzae, Moraxella catarrhalis, Staphylococcus aureus and/or Streptococcus pneumoniae. All 21 adenoid samples were culture positive for one or more of these four otopathogens. H. influenzae (35.3 %) and S. pneumoniae (76.2 %) were the most frequently cultured bacteria in the MEE and adenoid samples, respectively. The same bacterial species was found in MEE and adenoid for 84.6 % of the patients and in 81.2 % of the cases where the same species was found in more than one site it involved the same bacterial genotype. FISH and CLSM demonstrated the presence of H. influenzae specific biofilm structures in five of the eight culture positive MEEs that were tested, but in none of the two culture negative MEEs. The findings in this study indicate that the adenoid acts as a reservoir for bacteria in MEE and confirms that biofilms, in at least half of the cases consisting of H. influenzae, are indeed present in the MEE of children with COME. Biofilms may thus play a crucial role in the pathogenesis of COME, which is important in the understanding of this disease and the development of potential future treatment options.

Similar content being viewed by others
References
Dhooge I, Desloovere C, Boudewyns A, Van Kempen M, Dachy JP (2005) Management of otitis media with effusion in children. B-ENT Suppl 1:3–13
Williamson I (2011) Otitis media with effusion in children. BMJ Clin Evid pii:0502
Daniel M, Imtiaz-Umer S, Fergie N, Birchall JP, Bayston R (2012) Bacterial involvement in otitis media with effusion. Int J Ped ORL 76:1416–1422
Berkman ND, Wallace IF, Steiner MJ, Harrison M, Greenblatt AM, Lohr KN, Kimple A, Yuen A (2013) Otitis media with efusion: comparative effectiveness of treatments. AHRQ Comp Eff Rev 101:13
Khanna R, Lakhanpaul M, Bull PD, Guideline Development Group (2008) Surgical management of otitis media with effusion in children: summary of NICE guidance. Clin Otolaryngol 33:600–605
Post JC, Preston RA, Aul JJ, Larkins-Pettigrew M, Rydqvist-White J, Anderson KW (1995) Molecular analysis of bacterial pathogens in otitis media with effusion. JAMA 273:1598–1604
Rayner MG, Zhang Y, Gorry MC, Chen Y, Post JC, Ehrlich GD (1998) Evidence of bacterial metabolic activity in culture-negative otitis media with effusion. JAMA 279:296–299
Fergie N, Bayston R, Perason JP, Birchal JP (2004) Is otitis media with effusion a biofilm infection? Clin Otolaryngol 29:1598–1604
Gu X, Keyoumu Y, Long L, Zhang H (2014) Detection of bacterial biofilms in different types of chronic otitis media. Eur Arch Otorhinolaryngol 271:2877–2883
Høiby N, Bjarnsholt T, Givskov M, Molin S, Ciofu O (2010) Antibiotic resistance of bacterial biofilms. Int J Antimicrob Agents 35:322–332
Hall-Stoodley L, Hu FZ, Gieseke A, Nistico L, Nguyen D, Hayes J, Forbes M, Greenberg DP, Dice B, Burrows A, Wackym PA, Stoodley P, Post JC, Ehrlich GD, Kerschner JE (2006) Direct detection of bacterial biofilms on the middle-ear mucosa of children with chronic otitis media. JAMA 296:202–211
Dohar JE, Hebda PA, Veeh R, Awad M, Costerton JW, Hayes J, Ehrlich GD (2005) Mucosal biofilm formation on middle-ear mucosa in a nonhuman primate model of chronic suppurative otitis media. Laryngoscope 115:1469–1472
Chole RA, Faddis BT (2002) Evidence for microbial biofilms in cholesteatomas. Arch Otolaryngol Head Neck Surg 128:1129–1133
Saunders J, Murray M, Alleman A (2011) Biofilms in chronic suppurative otitis media and cholesteatoma: scanning electron microscopy findings. Am J Otolaryngol 32:32–37
Lampikoski H, Aarnalison AA, Jero J, Kinnari TJ (2012) Mastoid biofilm in chronic otitis media. Otol Neurotol 33:785–788
Thornton RB, Rigby PJ, Wiertsema SP, Filion P, Langlands J, Coates HL, Vijayasekaran S, Keil AD, Richmond PC (2011) Multi-species bacterial biofilm and intracellular infection in otitis media. BMC Pediatr. doi:10.1186/1471-2431-11-94
Corrazziari ES (2009) Intestinal mucus barrier in normal and inflamed colon. J Pediatr Gastroenterol Nutr 485(Suppl 2):S54–S55
Winther B, Gross BC, Hendley JO, Early SV (2009) Location of bacterial biofilm in the mucus overlying the adenoid by light microscopy. Arch Otolaryngol Head Neck Surg 135:1239–1245
Deschaght P, Van Simaey L, Decat E, Van Mechelen E, Brisse S, Vaneechoutte M (2011) Rapid genotyping of Achromobacter xylosoxidans, Acinetobacter baumannii, Klebsiella pneumoniae, Pseudomonas aeruginosa and Stenotrophomonas maltophilia isolates using melting curve analysis of RAPD-generated DNA fragments (McRAPD). Res Microbiol 162:386–392
Bluestone CD, Stephenson JS, Martin LM (1992) Ten-year review of otitis media pathogens. Pediatr Infect Dis J 11:S7–S11
Frickmann H, Christner M, Donat M, Berger A, Essig A, Podbielski A et al (2013) Rapid discrimination of Haemophilus influenzae, H. parainfluenzae, and H. haemolyticus by fluorescence in situ hybridization (FISH) and two matrix-assisted laser-desorption-ionization time-of-flight mass spectrometry (MALDI-TOF-MS) platforms. PLoS One 8:e63222
Amann R, Fuchs BM (2008) Single-cell identification in microbial communities by improved fluorescence in situ hybridization techniques. Nature Rev Microbiol 6:339–348
Bakaletz LO (2012) Bacterial biofilms in the upper airway—evidence for role in pathology and implications for treatment of otitis media. Paediatr Respir Rev 13:154–159
Gok U, Bulut Y, Keles E, Yalcin S, Doymaz MZ (2001) Bacteriological and PCR analysis of clinical material aspirated from otitis media with effusions. Int J Pediatr Otorhinolaryngol 60:49–54
Poetker DM, Lindstrom DR, Edmiston CE, Krepel CJ, Link TR, Kerschner JE (2005) Microbiology of middle ear effusions from 292 patients undergoing tympanostomy tube placement for middle ear disease. Int J Pediatr Otorhinolaryngol 69:799–804
Donlan RM (2001) Biofilm formation: a clinically relevant microbiological process. Clin Infect Dis 33:1387–1392
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The authors have no conflict of interest. No external funding was provided for this study. The study was approved by the Ethical Committee of Ghent University Hospital (number of approval: B670201214394). Written informed consent was provided by the parents or legal guardians of the study participants.
Electronic supplementary material
Below is the link to the electronic supplementary material.
405_2016_3958_MOESM2_ESM.avi
Figure S1. 3D animations of biofilm structures obtained by CLSM of FISH stained samplesAnimations were prepared with ImageJ software [29]. Legend. Two probes were used for the visualisation of biofilms in MEE samples: EUB388-Alexa555 probe is a universal probe which stains all bacteria green. The H. influenzae specific probe stains H. influenzae bacteria red. The combination of the EUB388-Alexa555 probe and the H. influenzae specific probe results in a yellow colour, which specifically indicates the presence of H. influenzae. DAPI (4’,6-diamidino-2-phenylindole) pigment stains eukaryote nucleoli blue. ORL1 MEE from right ear (AVI 1757 kb)
405_2016_3958_MOESM3_ESM.avi
Figure S1. 3D animations of biofilm structures obtained by CLSM of FISH stained samplesAnimations were prepared with ImageJ software [29]. Legend. Two probes were used for the visualisation of biofilms in MEE samples: EUB388-Alexa555 probe is a universal probe which stains all bacteria green. The H. influenzae specific probe stains H. influenzae bacteria red. The combination of the EUB388-Alexa555 probe and the H. influenzae specific probe results in a yellow colour, which specifically indicates the presence of H. influenzae. DAPI (4’,6-diamidino-2-phenylindole) pigment stains eukaryote nucleoli blue. ORL2 MEE from right ear (AVI 1913 kb)
405_2016_3958_MOESM4_ESM.avi
Figure S1. 3D animations of biofilm structures obtained by CLSM of FISH stained samplesAnimations were prepared with ImageJ software [29]. Legend. Two probes were used for the visualisation of biofilms in MEE samples: EUB388-Alexa555 probe is a universal probe which stains all bacteria green. The H. influenzae specific probe stains H. influenzae bacteria red. The combination of the EUB388-Alexa555 probe and the H. influenzae specific probe results in a yellow colour, which specifically indicates the presence of H. influenzae. DAPI (4’,6-diamidino-2-phenylindole) pigment stains eukaryote nucleoli blue. ORL12 MEE from left ear (first take) (AVI 1728 kb)
405_2016_3958_MOESM5_ESM.avi
Figure S1. 3D animations of biofilm structures obtained by CLSM of FISH stained samplesAnimations were prepared with ImageJ software [29]. Legend. Two probes were used for the visualisation of biofilms in MEE samples: EUB388-Alexa555 probe is a universal probe which stains all bacteria green. The H. influenzae specific probe stains H. influenzae bacteria red. The combination of the EUB388-Alexa555 probe and the H. influenzae specific probe results in a yellow colour, which specifically indicates the presence of H. influenzae. DAPI (4’,6-diamidino-2-phenylindole) pigment stains eukaryote nucleoli blue. ORL12 MEE from left ear (second take) (AVI 2488 kb)
Rights and permissions
About this article
Cite this article
Van Hoecke, H., De Paepe, AS., Lambert, E. et al. Haemophilus influenzae biofilm formation in chronic otitis media with effusion. Eur Arch Otorhinolaryngol 273, 3553–3560 (2016). https://doi.org/10.1007/s00405-016-3958-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00405-016-3958-9