Abstract
The hypertrophic adenoid may promote chronic suppurative otitis media in children as it fulfills its immune function. The number of lymphocytes in the adenoid and their cooperation in the immune response depend of on their proliferation and migration to the effector sites. Interleukin 7 (IL-7) is essential for the normal development and function lymphocytes. IL-7 plays pivotal role for activation and proliferation of T and B cells. The heterodimeric interleukin-7 receptor (IL-7R) is composed of the IL-7Rα (127) and the common cytokine receptor γc (CD132). The aim of this study was to evaluate the percentage of lymphocytes T (CD4+ and CD8+) with IL-7R (CD127 and CD132) expression in hypertrophic adenoid in children suffering with otitis media with effusion for a duration of 3 months. Adenoid excised due to hypertrophy with or without chronic otitis media with effusion was used as study material. CD4+ CD127+, CD4+132+, CD8+CD127+ and CD8+CD132+ cell subpopulations were identified using monoclonal antibodies and flow cytometry. The percentage of CD4+ and CD8+ T cells with CD127 receptor expression in hypertrophic adenoid of children with otitis media with effusion was statistically significantly higher than in hypertrophic adenoid group. The percentage of CD4+ T cells with CD132 expression in the study group was statistically significantly higher than in the reference group. The percentage of CD8+ T cells with CD132+ expression was not statistically different in both groups. The increased percentage of T lymphocytes with IL-7R expression (CD127 and CD132) in hypertrophic adenoid seems to influence the quantity of lymphocytes and upset the immunological function of tonsils which can influence the course of otitis media with effusion.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Interleukin 7 (IL-7) plays a basic role in the development of lymphocytes in the thymus, in the production of memory T cells and in the homeostasis of peripheral T cells [1–3]. IL-7 is generated by nonhematopoietic stromal cells of many organs, including the thymus, bone marrow and peripheral lymphatic organs [2, 3].
The receptor for IL-7 (IL-7R) is a heterodimer composed of α chain (IL-7Rα or CD127) and the accompanying γ chain (γc or CD132), which is also a receptor for IL-2, IL-4, IL-9, IL-15 and IL-21 [1, 2, 4–6]. The expression of IL-7R (CD127 and CD132) can be found in naive CD4+/CD8+ T cells and CD4+/CD8+ memory T cells [7–10]. IL-7 binding to the receptor results in the initiation of at least three activation cascades: JAK/STAT (Janus kinase/signal transducer and activator of transcription), PI3 K (Phosphoinositide 3-kinase) and MAPK (mitogen-activated protein kinase)/ERK (extracellular signal-related kinase) [5, 11, 12].
The signal transmitted through the IL-7Rα induces increased expression of apoptotic proteins, mainly Bcl-2 [10, 12–14], cell proliferation [13, 15], antigen-independent naive T-cell proliferation, strong expansion of memory T cells (effector) in the presence and lack of antigen [7, 8, 16]. The IL-7Rα also plays an important role in signal transmission required for the development of the secondary lymphoid system [17]. At the same time it stimulates Fas antigen expression, by increasing T cell sensitivity to apoptosis [18].
The regulation of IL-7Rα expression is of major importance for the efficient production of CD4+ and CD8+ memory cells, as a result of the immune response. The basic level of IL-7 is sufficient for the maintenance of viability of CD8+ memory cells and to some extent contributes to the maintenance of viability of the CD4+ memory T cell pool [2].
The study objective was to assess the percentage of lymphocytes T (CD4+ and CD8+) with IL-7R (CD127 and CD132) expression in the group of children with hypertrophic adenoid in children with otitis media with effusion who suffer of 3 months’ duration.
Materials and methods
The study material contained adenoid excised from 35 children (18 girls and 17 boys, aged from 3 to 17) suffering from adenoid hypertrophy coexisting with otitis media with effusion (OME) lasting at least 3 months. The reference group consisted of 30 children (15 girls and 15 boys, aged from 3 to 17) with adenoid hypertrophy (HA), but without otitis media, lasting at least 3 months. All children were clinically free of infection at the time of surgery. Due the treatment failure, the children were qualified for adenoidectomy in the Department of Children’s Otolaryngology Medical University of Bialystok. The study was approved by the Bioethical Committee, Medical University of Bialystok (number R-I -002/264/2009).
Methods
Immediately after excision, the adenoids were placed in RPMI 1640 culture medium containing 10% fetal calf serum (Immuniq). Then, they underwent mechanical grinding so as to give a homogenous suspension. The suspension was centrifuged for 1 min. at 4°C (at 100–150×g). The obtained supernatant containing leukocytes was subjected to further analysis. Cells were obtained from the supernatant through centrifugation for 8 min at 4°C (at 300×g), and then rinsed with PBS containing 0.1 mM EDTA and 0.02% NaN3 (Sigma) and centrifuged again for 8 min at 4°C (at 300×g).
The density of cell suspension and cell morphology was assessed both under a microscope and using a hematologic analyzer Sysmex XT 2000i. Cell suspension of 4–10 × 103/μl density was used for analysis. The isolated mononuclear cells in the form of suspension in PBS were incubated with monoclonal antibodies conjugated with fluorochromes directed against antigens (CD4-PE-Cy5, CD8-FITC, CD4-FITC/CD8PE/CD3-PerCP, CD127-PE, CD132-PE) (Beckman Coulter). The samples were evaluated in a flow cytometer Coulter PC500, equipped with argon laser emitting at 488 nm.
Statistical analysis
Non-parametric U Mann–Whitney test was used for statistical analysis. The differences for p < 0.05 were considered statistically significant. The results were described as the mean, standard deviation, median, minimum and maximum values and lower P 25 and upper P 75 quartiles.
Results
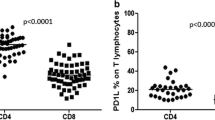
The mean percentage of CD4+ T cells with CD127 expression in hypertrophic adenoid with otitis media with effusion (OME) was 56.37 ± 5.73%, being statistically significantly higher (p < 0.005) than in the reference group (HA 48.18 ± 9.41%) (Table 1). In OME group, the median of the percentage of CD4+CD127+ lymphocytes was 57.05%, P 25 53.60% and P 75 61.00%, with result range of 44.20–63.80%. The confidence interval for the percentage of CD4+CD127+ lymphocytes in the reference group (HA) ranged from 45.20% (P 25) to 54.80% (P 75), median 49.65% and result range of 27.30–67.60% (Fig. 1).
The mean percentage of CD8+ lymphocytes with CD127 expression in OME children was 56.53 ± 11.87%, being statistically significantly higher (p < 0.04) than in reference group (HA 48.72 ± 9.03%) (Table 1). In the study group, the median of the percentage of CD8+CD127+ lymphocytes was 55.6%, with P 25 48.80% and P 75 65.90% and the range of results 37.70–80.00%. The confidence interval for the percentage of CD8+CD127+ lymphocytes in the reference group (HA) ranged from 40.60% (P 25) to 62.50% (P 75) with the median equal to 49.00% and the range of results from 34.10 to 67.60% (Fig. 2).
The mean percentage of CD4+ lymphocytes with CD132 expression in hypertrophic adenoid in children with otitis media with effusion was 45.44 ± 8.13%, being statistically significantly higher (p < 0.05) than in the reference group (HA 39.91 ± 7.54%). In the study group (OME), the median of the percentage of CD4+CD132+ cells was 46.20%, P 25 39.90% and P 75 49.00%, with the range of results from 31.80 to 61.80%. The confidence interval for the percentage of CD4+CD132+ lymphocytes in the reference group (HA) ranged between 39.40% (P 25) and 45.30% (P 75), median 40.80% and the range of results from 22.90 to 50.00% (Fig. 3).
The mean percentage of CD8+ T cells with CD132 expression in OME children was 50.48 ± 11.75%, being higher than in the reference group (44.6 ± 9.21%). The difference was not statistically significant (Table 1). In the study group (OME), the median of the percentage of CD8+CD132+ cells was 50.75%, P 25 39.10%, P 75 59.80% and the range of results from 34.80 to 74.40%. The confidence interval for the percentage of CD8+CD132+ cells in the reference group ranged from 39.90% (P 25) to 62.50% (P 75), median 44.25% and the range of results between 27.00 and 58.80% (Fig. 4).
Discussion
The interfollicular space of the adenoid is dominated by T cells, both naive and memory lymphocytes, which get there through high endothelial venule (HEV) from peripheral blood. Naive T cells, after penetrating the interfollicular space of the adenoid, get into contact with antigen-presenting cells (APC), which eventually leads to their reactivation, retention in the interfollicular space and proliferation [19]. Some of the naive T cells are converted into effector cells which undergo rapid elimination after pathogen destruction, whereas others into long-lived memory cells [20].
Factors that affect T cell viability vary depending on the state of these cells [21]. One of the major factors is pleiotropic IL-7, produced by stromal cells of many organs [2, 4], as well as by dendritic cells in the peripheral lymphatic organs [22]. Fry et al. [4] claim that since IL-7 is produced by dendritic cells of the peripheral lymphatic organs and T cell response to IL-7 is IL-7R expression dependent, a reduction in the number of lymphocytes does not cause their dysfunction [23].
The percentage of CD4+ and CD8+ T cells with CD127 receptor expression in hypertrophic adenoid in children with otitis media with effusion was statistically significantly higher than in HA group. The percentage of CD4+ T cells with CD132 expression in the study group was statistically significantly higher than in the reference group. However, the percentage of CD8+CD132+ T cells in the study group was only slightly higher compared with the reference group.
IL-7 is known to regulate T cell viability. When bound to its receptor (CD127) on CD8+ lymphocytes it increases the expression of anti-apoptotic proteins and induces the conversion of effector cells into long-lived memory cells [10]. On the other hand, Fluur et al. [18] have shown that IL-7 also stimulates Fas expression and its level correlates with T cell sensitivity to apoptosis. Previously, we have demonstrated that lymphocyte apoptosis in hypertrophic adenoid in children with otitis media with effusion more enhanced than in the HA group, which seems to be due to the fact that in hypertrophic adenoid of OME children the percentage of CD4+ and CD8+ lymphocytes with Fas expression was higher compared with the group without the inflammation, whereas the percentage of these cells with Bcl-2 expression was lower [3].
As IL-7 takes part in the regeneration of T cells, their increased sensitivity to this cytokine is essential for the maintenance of homeostasis. Previously, we have found that hypertrophic adenoid of children with otitis media with effusion exhibits markedly lower percentages of CD4+ and CD8+ T cells [24]. Therefore, a high percentage of T cells with IL-7R expression in children with otitis media with effusion may contribute to the maintenance of normal proportions in the subpopulations of adenoid T cells. At the same time it may lead to the formation of a considerable number of memory cells.
Conclusion
The increased percentage of T lymphocytes with IL-7R expression (CD127 and CD132) in hypertrophic adenoid seems to influence the quantity of lymphocytes and upset the immunological function of tonsils which can influence the course of otitis media with effusion.
References
Fry TJ, Mackall CL (2001) Interleukin -7: master regulator of peripheral T-cell homeostasis. Trend Immunol 22(10):564–571
Fry TJ, Mackall CL (2005) The many faces of IL-7, from lymphopoiesis to peripheral T cell maintenance. J Immunol 174(11):6571–6576
Żelazowska-Rutkowska B, Wysocka J, Skotnicka B (2010) Chosen factors of T and B cell apoptosis in hypetrophic adenoid in children with otitis media effusion. Int J Pediatr Otorhinolaryngol 74(6):698–700
Fry TJ, Connick E, Faloon J, Lederman MM, Liewehr DJ, Spitzler J, Wood LV, Yorchoan R, Zuckerman J, Landau A, Mackall CLA (2001) potential role for interleukin 7 in T cell homeostasis. Blood 97(10):2983–2990
Giliani S, Mori L, de Saint Basile G, Le Deist F, Rodriguez-Perez C, Forrino C, Mazzolani E, Dupuis S, Elhasid R, Kessel A, Galambrun C, Gil J, Fischer A, Etzioni A, Notarangelo LD (2005) Interleukin-7 receptor α (IL-Rα) deficiency: cellular and molecular bases. Analysis of clinical, immunological, and molecular features in 16 novel patients. Immunol Rev 203:110–126
Ziegler SE, Morella KK, Anderson D, Kumaki N, Leonard WJ, Cosman D, Baumann H (2005) Reconstitution of a functional interleukin (IL)-7 receptor demonstrates that the IL-2 receptor gamma chain is required for IL-7 signal transduction. Eur J Immunol 25(2):399–404
Tan JT, Dudi E, LoRoy E, Murney R, Sprent J, Weinberg KI, Surh CD (2001) IL-7 is critical for homeostatic proliferation and survival of naive T cells. PNAS 98(15):8732–8737
Schluns KS, Kieper WC, Jameson SC, Lefrancois L (2000) Interleukin-7 mediates the homeostasis of naive and memory CD8 T cells in vivo. Nat Immunol 1(5):426–432
Kaech SM, Tan JT, Wherry EJ, Konieczny BT, Surh CD, Ahmed R (2003) Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat Immunol 4(12):1191–1198
Kondrack RM, Harbertson J, Tan JT, McBreen ME, Surh CD, Bradley LM (2003) Interleukin 7 regulates the survival and generation of memory cells. J Exp Med 198(12):1797–1806
Benczik M, Gaffen SL (2004) The interleukin (IL)-2 family cytokines :survival and proliferation signaling pathways in T lymphocytes. Immunol Invest 33(2):109–142
Chetoui N, Boisvert M, Gendron S, Aoudjit F (2010) Interleukin-7 promotes the survival of human CD4+ effector/memory T cells by up-regulating Bcl-2 proteins and activating the JAK/STAT signaling pathway. Immunology 130(3):418–426
Dereuddre-Bosquet N, Vaslin B, Delache B, Brochrd P, Clayette P, Aubenque C, Morre M, Assouline B, Le Grand R (2007) Rapid modifications of peripheral T-cell subsets that Express CD127 in macaques treated with recombinant IL-7. J Med Primatol 36(4–5):228–237
Sportes C, Hakim FT, Memon SA, Zhang H, Chua KS, Brown MR, Flescher TA, Krumlauf MC, Babb RR, Chow CK, Fry T, Engel SJ, Buffet R, Morre M, Amato RJ, Kenzon DJ, Korungold R, Pecora A, Gress RE, Mackall CL (2008) Administration of rhIL-7 in humans increases in vivo TCR repertoire diversity by preferential expansion of naive T cell subsets. J Exp Med 205(7):1701–1714
Bosco N, Agenes F, Ceredig R (2005) Effects of increasing IL-7 avaiability on lymphocytes during and after lymphopenia-induced proliferation. J Immunol 175(1):25–26
Ramsey C, Rubinstein MP, Kim DM, Cho JH, Sprent J, Surh CD (2008) The lymphopenic environment of CD132 (common γ-chain)-deficient hosts elicits rapid homeostastic proliferation of naive T cells via IL-15. J Immunol 180(8):5320–5326
Vudattu NK, Magalhaes I, Schmidt M, Seyfert-Margolis V, Maeurer MJ (2007) Reduced numbers of IL-7 receptor (CD127) ex pressing immune cells and IL-7-signalling defects in peripheral blood of patients with breast cancer. Int J Cancer 121(7):1512–1519
Fluur C, De Milito A, Fry TJ, Vivar N, Eidsmo L, Atlas A, Federici C, Matarrese P, Logozzi M, Rajnavolgi E, Mackall CL, Fais S, Chiodi F, Rethi B (2007) Potential role for IL-7 in Fas-mediatel T cell apoptosis during HIV infection. J Immunol 178(8):5340–5350
Van Kempen MJP, Rijkers GT, Van Cauwenberge PB (2000) The immune response in adenoids and tonsils. In. Arch Allergy Immunol 122(1):8–19
Boyman O, Letourneaou S, Krieg C, Sprent J (2009) Homeostatic proliferation and survival of naïve and T memory cells. Eur J Immunol 39(8):2088–2094
Marrack P, Kappler J (2004) Control of T cell viability. Annu Rev Immunol 22:765–787
Kondrack RM, Harbertson J, Tan JT, McBreen ME, Surh CD, Bradley LM (2003) Interleukin 7 regulates the survival and generation of memory cells. J Exp Med 198(12):1797–1806
Rosenberg SA, Sportes C, Ahmadzadeh M, Fry TJ, Ngo LT, Schwartz SL, Stetler-Stevenson M, Marton KE, Mavronkakis SA, Mome M, Buffet R, Mackall CL, Gress RE (2006) IL-7 administration to humans leads to expansion of CD8 + and CD4 + T regulatory cells. J Immunother 29(3):313–319
Wysocka J, Hassmann-Poznańska E, Kasprzycka E, Musiatowicz M (2001) Lymphocyte subpopulations in the adenoids analyzed by flow cytometry. Wiad Lek 54(7–8):418–423
Open Access
This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 2.0 International License (https://creativecommons.org/licenses/by/2.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Żelazowska-Rutkowska, B., Wysocka, J., Ratomski, K. et al. Increased percentage of T cells with the expression of CD127 and CD132 in hypertrophic adenoid in children with otitis media with effusion. Eur Arch Otorhinolaryngol 269, 1821–1825 (2012). https://doi.org/10.1007/s00405-012-1977-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00405-012-1977-8