Abstract
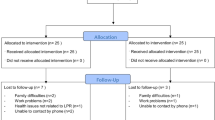
Laryngopharyngeal reflux (LPR) refers to the backflow of stomach contents into the laryngopharynx. Increasing evidence has demonstrated that LPR is a contributing factor in some cases of hoarseness, vocal fatigue, voice breaks, cough and globus and chronic throat clearing. However, several randomised placebo-controlled trials of proton pump inhibitors in the treatment of LPR have been reported with the majority showing no significant benefit in patient symptom scores over placebo. The aim of this pilot clinical study was to investigate whether any improvement in LPR-related symptoms, using the Reflux Symptom Index (RSI), and clinical findings, using the Reflux Finding Score (RFS), could be achieved with treatment with a liquid alginate suspension compared to control (no treatment). Patients presenting with the symptoms of LPR to the Otorhinolaryngology Outpatient Department at the Queen’s Medical Centre, Nottingham, UK were considered eligible if they had an RSI of greater than 10 and an RFS greater than 5 based on a fibreoptic examination of the larynx. A total of 49 patients were randomised into the open, parallel group study; 24 patients were randomised to receive 10 ml liquid alginate suspension (Gaviscon® Advance) four times daily after meals and at bedtime, and 25 patients into the control group (no treatment). Patients were assessed pre-treatment and at 2, 4 and 6 months post treatment. Mean (SD) RSI and RFS pre-treatment scores were 23.9 (7.0) and 10.4 (3.6) for the treatment group and 24.6 (7.4) and 10.3 (3.3) for the control group, respectively. Significant differences between treatment and control were observed for RSI at the 2-month (11.2 (7.0) vs. 16.8 (6.4), P = 0.005) and 6-month (11.2 (8.1) vs. 18.3 (9.4), P = 0.008) assessments and for RFS at the 6-month (7.1 (2.8) vs. 9.5 (3.4), P = 0.005) assessment. Significant improvement in symptom scores and clinical findings were achieved with liquid alginate suspension (Gaviscon® Advance) compared to control and further evaluation for the management of patients presenting with LPR is warranted.
Similar content being viewed by others
Abbreviations
- ANCOVA:
-
Analysis of covariance
- FDA:
-
Food and Drug Administration
- GCP:
-
Good clinical practice
- GORD:
-
Gastro-oesophageal reflux disease
- GOR:
-
Gastro-oesophageal reflux
- ICH:
-
International conference on harmonisation
- ITT:
-
Intention to treat
- LOCF:
-
Last observation carried forward
- LPR:
-
Laryngopharyngeal reflux
- PPI:
-
Proton pump inhibitor
- RFS:
-
Reflux Finding Score
- RSI:
-
Reflux Symptom Index
- SD:
-
Standard deviation
References
Copper MP, Smit CF, Stanojcic LD, Devriese PP, Schouwenburg PF, Mathus-Vliegen LM (2000) High incidence of laryngopharyngeal reflux in patients with head and neck cancer. Laryngoscope 110:1007–1011. doi:10.1097/00005537-200006000-00023
Chen MY, Ott DJ, Casolo BJ, Moghazy KM, Koufman JA (1998) Correlation of laryngeal and pharyngeal carcinomas and 24-hour pH monitoring of the esophagus and pharynx. Otolaryngol Head Neck Surg 119:460–462
Koufman JA (1991) The otolaryngologic manifestations of gastroesophageal reflux disease (GERD): a clinical investigation of 225 patients using ambulatory 24-hour pH monitoring and an experimental investigation of the role of acid and pepsin in the development of laryngeal injury. Laryngoscope 101:1–78
Ossakow SJ, Elta G, Colturi T, Bogdasarian R, Nostrant TT (1987) Esophageal reflux and dysmotility as the basis for persistent cervical symptoms. Ann Otol Rhinol Laryngol 96:387–392
Koufman JA, Amin MR, Panetti M (2000) Prevalence of reflux in 113 consecutive patients with laryngeal and voice disorders. Otolaryngol Head Neck Surg 123:385–388. doi:10.1067/mhn.2000.109935
Belafsky PC, Postma GN, Koufman JA (2002) Validity and reliability of the reflux symptom index (RSI). J Voice 16:274–277. doi:10.1016/S0892-1997(02)00097-8
Belafsky PC, Postma GN, Koufman JA (2001) Laryngopharyngeal reflux symptoms improve before changes in physical findings. Laryngoscope 111:979–981. doi:10.1097/00005537-200106000-00009
Belafsky PC, Postma GN, Koufman JA (2001) The validity and reliability of the reflux finding score (RFS). Laryngoscope 111:1313–1317. doi:10.1097/00005537-200108000-00001
Richter JE, Hicks DM (1997) Unresolved issues in gastroesophageal reflux-related ear, nose, and throat problems. Am J Gastroenterol 92:2143–2144
Postma GN (2000) Ambulatory pH monitoring methodology. Ann Otol Rhinol Laryngol Suppl 184:10–14
Vaezi MF, Schroeder PL, Richter JE (1997) Reproducibility of proximal probe pH parameters in 24-hour ambulatory esophageal pH monitoring. Am J Gastroenterol 92:825–829
Willems-Bloemer LH, Vreeburg GC, Brummer R (2000) Treatment of reflux-related and non-reflux-related dysphonia with profound gastric acid inhibition. Folia Phoniatr Logop 52:289–294. doi:10.1159/000021546
Freston JW (1997) Long-term acid control and proton pump inhibitors: interactions and safety issues in perspective. Am J Gastroenterol 92:51S–55S; discussion 55S–57S
FDA (2007) Early communication about an ongoing safety review: omeprazole (Prilosec), esomeprazole (Nexium)
Noordzij JP, Khidr A, Evans BA, Desper E, Mittal RK, Reibel JF, Levine PA (2001) Evaluation of omeprazole in the treatment of reflux laryngitis: a prospective, placebo-controlled, randomized, double-blind study. Laryngoscope 111:2147–2151. doi:10.1097/00005537-200112000-00013
El-Serag HB, Lee P, Buchner A, Inadomi JM, Gavin M, McCarthy DM (2001) Lansoprazole treatment of patients with chronic idiopathic laryngitis: a placebo-controlled trial. Am J Gastroenterol 96:979–983. doi:10.1111/j.1572-0241.2001.03681.x
Qua CS, Wong CH, Gopala K, Goh KL (2007) Gastro-oesophageal reflux disease in chronic laryngitis: prevalence and response to acid-suppressive therapy. Aliment Pharmacol Ther 25:287–295
Vaezi MF, Richter JE, Stasney CR, Spiegel JR, Iannuzzi RA, Crawley JA, Hwang C, Sostek MB, Shaker R (2006) Treatment of chronic posterior laryngitis with esomeprazole. Laryngoscope 116:254–260. doi:10.1097/01.mlg.0000192173.00498.ba
Wo JM, Koopman J, Harrell SP, Parker K, Winstead W, Lentsch E (2006) Double-blind, placebo-controlled trial with single-dose pantoprazole for laryngopharyngeal reflux. Am J Gastroenterol 101:1972–1978. doi:10.1111/j.1572-0241.2006.00693.x
Eherer AJ, Habermann W, Hammer HF, Kiesler K, Friedrich G, Krejs GJ (2003) Effect of pantoprazole on the course of reflux-associated laryngitis: a placebo-controlled double-blind crossover study. Scand J Gastroenterol 38:462–467. doi:10.1080/00365520310001860
Steward DL, Wilson KM, Kelly DH, Patil MS, Schwartzbauer HR, Long JD, Welge JA (2004) Proton pump inhibitor therapy for chronic laryngo-pharyngitis: a randomized placebo-control trial. Otolaryngol Head Neck Surg 131:342–350. doi:10.1016/j.otohns.2004.03.037
Gatta L, Vaira D, Sorrenti G, Zucchini S, Sama C, Vakil N (2007) Meta-analysis: the efficacy of proton pump inhibitors for laryngeal symptoms attributed to gastro-oesophageal reflux disease. Aliment Pharmacol Ther 25:385–392
Ours TM, Kavuru MS, Schilz RJ, Richter JE (1999) A prospective evaluation of esophageal testing and a double-blind, randomized study of omeprazole in a diagnostic and therapeutic algorithm for chronic cough. Am J Gastroenterol 94:3131–3138. doi:10.1111/j.1572-0241.1999.01504.x
Vaezi MF, Hicks DM, Abelson TI, Richter JE (2003) Laryngeal signs and symptoms and gastroesophageal reflux disease (GERD): a critical assessment of cause and effect association. Clin Gastroenterol Hepatol 1:333–344. doi:10.1053/S1542-3565(03)00177-0
Tack J (2006) Review article: the role of bile and pepsin in the pathophysiology and treatment of gastro-oesophageal reflux disease. Aliment Pharmacol Ther 24:10–16. doi:10.1111/j.1365-2036.2006.03070.x
Washington N (1990) Investigation into the barrier action of an alginate gastric reflux suppressant, Liquid Gaviscon. Drug Invest 2:23–30
Author information
Authors and Affiliations
Corresponding author
Additional information
Disclosures: Peter Dettmar and Vicki Strugala are consultants to Reckitt Benckiser Healthcare. Lesley Johnstone and John Sykes are employees of Reckitt Benckiser Healthcare. This research was funded by Reckitt Benckiser Healthcare (UK) Ltd.
Rights and permissions
About this article
Cite this article
McGlashan, J.A., Johnstone, L.M., Sykes, J. et al. The value of a liquid alginate suspension (Gaviscon Advance) in the management of laryngopharyngeal reflux. Eur Arch Otorhinolaryngol 266, 243–251 (2009). https://doi.org/10.1007/s00405-008-0708-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00405-008-0708-7